Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter28: High-performance Liquid Chromatography
Section: Chapter Questions
Problem 28.19QAP
Related questions
Question
100%
Can someone check my answers and see if they are correct?
Transcribed Image Text:© Macmillan Learn
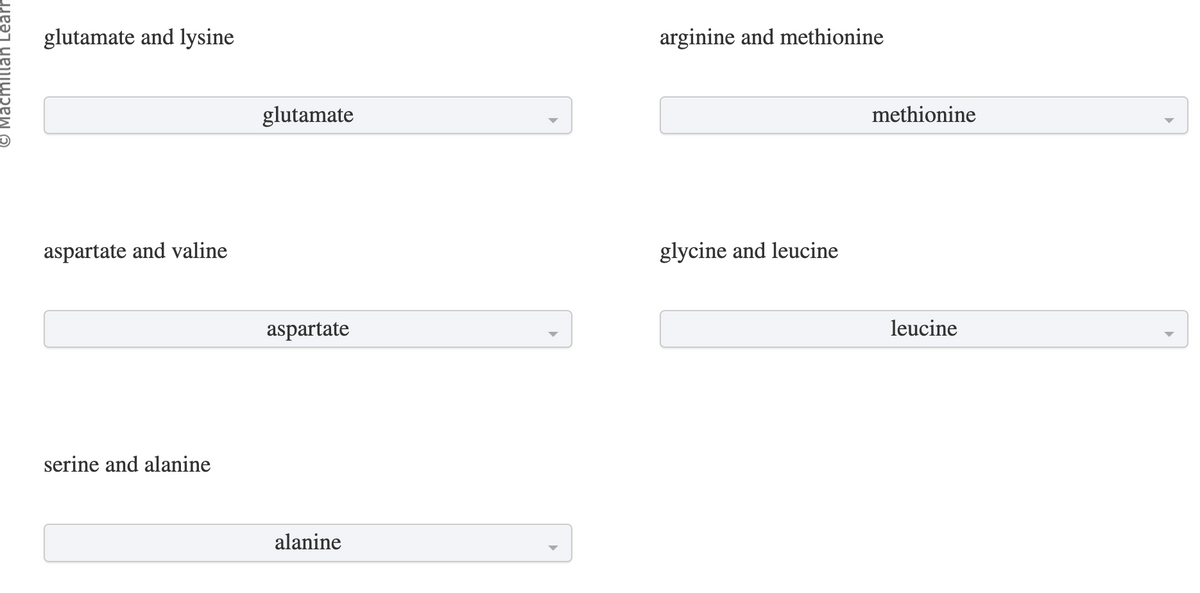
glutamate and lysine
aspartate and valine
serine and alanine
glutamate
aspartate
alanine
▼
arginine and methionine
glycine and leucine
methionine
leucine
Transcribed Image Text:O Macmillan Learning
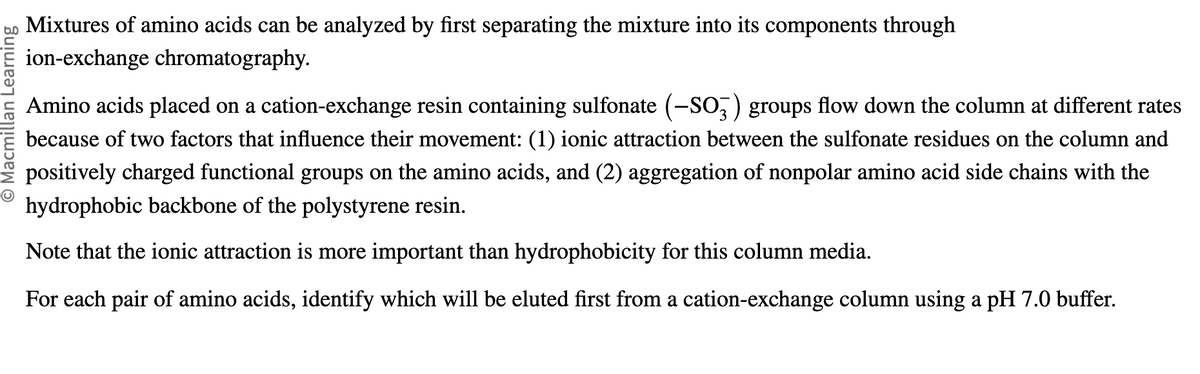
Mixtures of amino acids can be analyzed by first separating the mixture into its components through
ion-exchange chromatography.
Amino acids placed on a cation-exchange resin containing sulfonate (-SO3) groups flow down the column at different rates
because of two factors that influence their movement: (1) ionic attraction between the sulfonate residues on the column and
positively charged functional groups on the amino acids, and (2) aggregation of nonpolar amino acid side chains with the
hydrophobic backbone of the polystyrene resin.
Note that the ionic attraction is more important than hydrophobicity for this column media.
For each pair of amino acids, identify which will be eluted first from a cation-exchange column using a pH 7.0 buffer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
