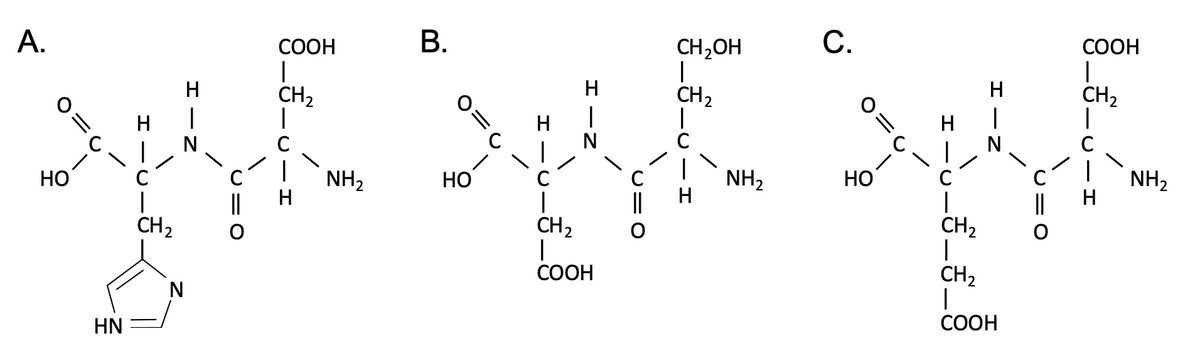
Imagine you are attempting to separate these three dipeptides from each other using anion exchange chromatography, and you use a protein assay to identify at what salt concentration each of them is eluted from the column. A graph showing protein concentration as a function of salt concentration is shown below the dipeptides. Which of the three dipeptides is represented by peak #3? (NOTE: the dipeptides are shown in their non-ionized forms. You must assume that they are in aqueous solution at physiological pH, ~7.2) a) Dipeptide A b) Dipeptide B c) Dipeptide C
Imagine you are attempting to separate these three dipeptides from each other using anion exchange chromatography, and you use a protein assay to identify at what salt concentration each of them is eluted from the column. A graph showing protein concentration as a function of salt concentration is shown below the dipeptides. Which of the three dipeptides is represented by peak #3? (NOTE: the dipeptides are shown in their non-ionized forms. You must assume that they are in aqueous solution at physiological pH, ~7.2) a) Dipeptide A b) Dipeptide B c) Dipeptide C
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 57QRT
Related questions
Question
Imagine you are attempting to separate these three dipeptides from each other using anion exchange chromatography, and you use a protein assay to identify at what salt concentration each of them is eluted from the column. A graph showing protein concentration as a function of salt concentration is shown below the dipeptides. Which of the three dipeptides is represented by peak #3? (NOTE: the dipeptides are shown in their non-ionized forms. You must assume that they are in aqueous solution at physiological pH, ~7.2)
a) Dipeptide A
b) Dipeptide B
c) Dipeptide C
![3
0 10 20 30 40 50
[NACI] (mM)
A260
2.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F89d4e4fb-1f7e-4614-824b-fd107892a18d%2F09b6c6ac-e97e-4141-b589-e42984ac526d%2Fx61xi0l_processed.png&w=3840&q=75)
Transcribed Image Text:3
0 10 20 30 40 50
[NACI] (mM)
A260
2.
Transcribed Image Text:В.
С.
СООН
CH,OH
|
CH2
А.
СООН
H.
H
CH2
CH2
O:
H
|
H
C
HO
|
NH2
НО
C
NH,
НО
|
CH2
NH2
H
||
CH2
||
CH2
СООН
CH2
СООН
HN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning