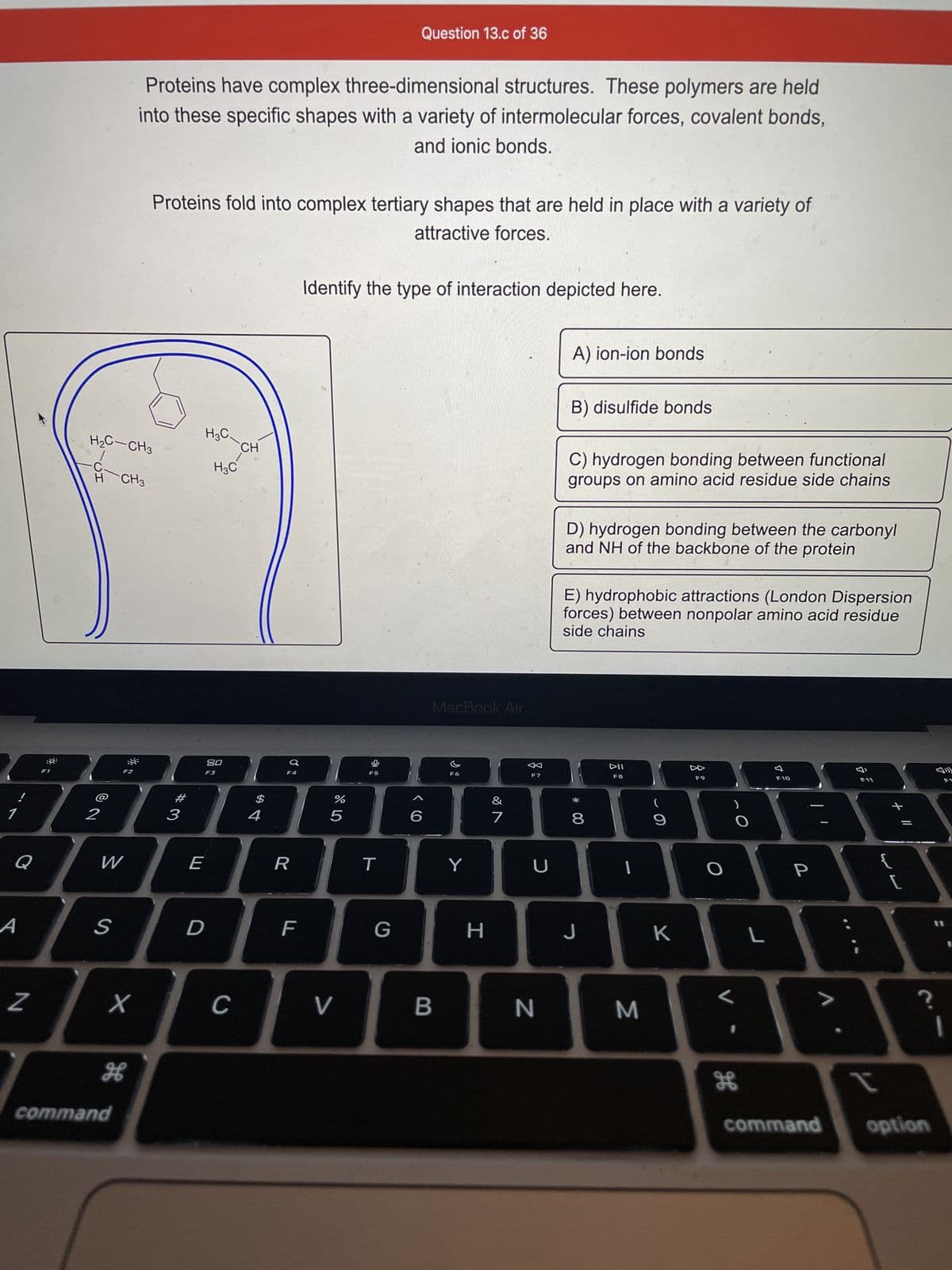
Proteins have complex three-dimensional structures. These polymers are held into these specific shapes with a variety of intermolecular forces, covalent bonds, and ionic bonds. Proteins fold into complex tertiary shapes that are held in place with a variety of attractive forces. H₂C-CH3 CH3 H3C H₂C CH Identify the type of interaction depicted here. A) ion-ion bonds B) disulfide bonds C) hydrogen bonding between functional groups on amino acid residue side chains D) hydrogen bonding between the carbonyl and NH of the backbone of the protein E) hydrophobic attractions (London Dispersion forces) between nonpolar amino acid residue side chains
Proteins have complex three-dimensional structures. These polymers are held into these specific shapes with a variety of intermolecular forces, covalent bonds, and ionic bonds. Proteins fold into complex tertiary shapes that are held in place with a variety of attractive forces. H₂C-CH3 CH3 H3C H₂C CH Identify the type of interaction depicted here. A) ion-ion bonds B) disulfide bonds C) hydrogen bonding between functional groups on amino acid residue side chains D) hydrogen bonding between the carbonyl and NH of the backbone of the protein E) hydrophobic attractions (London Dispersion forces) between nonpolar amino acid residue side chains
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 48QAP
Related questions
Question
Please solve these two
Transcribed Image Text:1
T
2
Q
A
:8:
N
H₂C-CH3
W
S
CH3
Proteins have complex three-dimensional structures. These polymers are held
into these specific shapes with a variety of intermolecular forces, covalent bonds,
and ionic bonds.
X
H
command
Proteins fold into complex tertiary shapes that are held in place with a variety of
attractive forces.
# 3
E
H3C.
H3C
80
F3
D
C
CH
$
4
F4
R
F
LL
Identify the type of interaction depicted here.
%
5
V
Question 13.c of 36
F5
T
G
6
MacBook Air
B
F6
Y
H
&
7
K
F7
U
N
A) ion-ion bonds
B) disulfide bonds
C) hydrogen bonding between functional
groups on amino acid residue side chains
D) hydrogen bonding between the carbonyl
and NH of the backbone of the protein
E) hydrophobic attractions (London Dispersion
forces) between nonpolar amino acid residue
side chains
* 00
8
J
DII
F8
1
M
9
K
F9
L
F10
P
7
F11
1
+ 11
command option
1
Transcribed Image Text:Tube
Maps
1
tion
A
F1
Q
+
Z
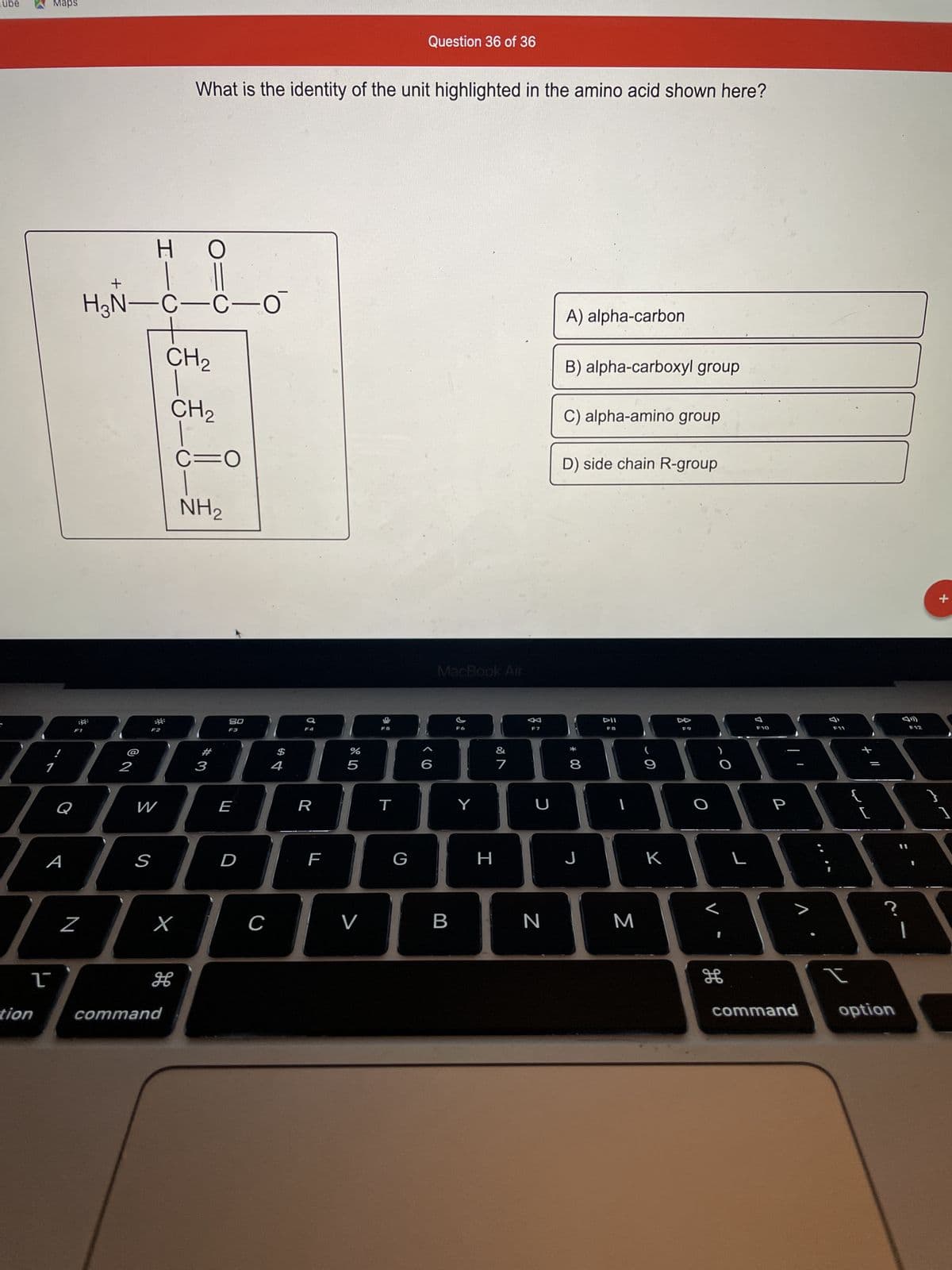
H₂N-C-C-0
2
HIC
F2
W
Н O
#
HAGAH
3
E
S
What is the identity of the unit highlighted in the amino acid shown here?
CH₂
CH ₂
command
X
H
C=O
NH₂
80
D
C
$
F4
4
R
F
%
5
V
T
Question 36 of 36
G
<C
MacBook Air
B
Y
H
V lº
&
7
K
F7
U
N
A) alpha-carbon
B) alpha-carboxyl group
C) alpha-amino group
D) side chain R-group
* 00
8
J
DII
FB
M
9
K
FO
H
L
F10
P
>
command
F11
I
لاب
L
option
}
+
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning