Carbon monoxide is poisonous because it binds to Hb in a similar fashion and can displace oxygen and block O2 from binding to Hb. a) The reaction below describes the equilibrium for the displacement of Oz in the HbO2 complex by CO to form HBCO. Write an equilibrium expression for this reaction. HbO2 + CO HBCO + O2 Kdisp = b) The equations below describe the equilibrium for O2 and CO binding to Hb. Using the information provided determine the value of Kdisp Hb +O2 HbO2 [HbO2] = 3.2 [Hb][O2] Ko2 = НЬ + СО HBCO [H6C0] [Hb][CO] Kco = = 750
Hemoglobin (Hb), carries oxygen from our lungs to the rest of out body. The figure below shows oxygen binding to the active site of Hb.
O
NON Fe NN
N N
Carbon monoxide is poisonous because it binds to Hb in a similar fashion and can displace oxygen and block O2 from binding to Hb.
a) The reaction below describes the equilibrium for the displacement of O2 in the HbO2 complex by CO to form HbCO. Write an equilibrium expression for this reaction.
HbO2 + CO HbCO + O2
Kdisp = ______________
b) The equations below describe the equilibrium for O2 and CO binding to Hb. Using the information provided determine the value of Kdisp
Hb +O2 HbO2
?"#= [???#]=3.2 [??][?#]
Hb + CO
HbCO
?$"= [????]=750 [??][??]
16
Initials __________
c) Use your Kdisp to calculate ΔGo for the displacement reaction at 298 K.
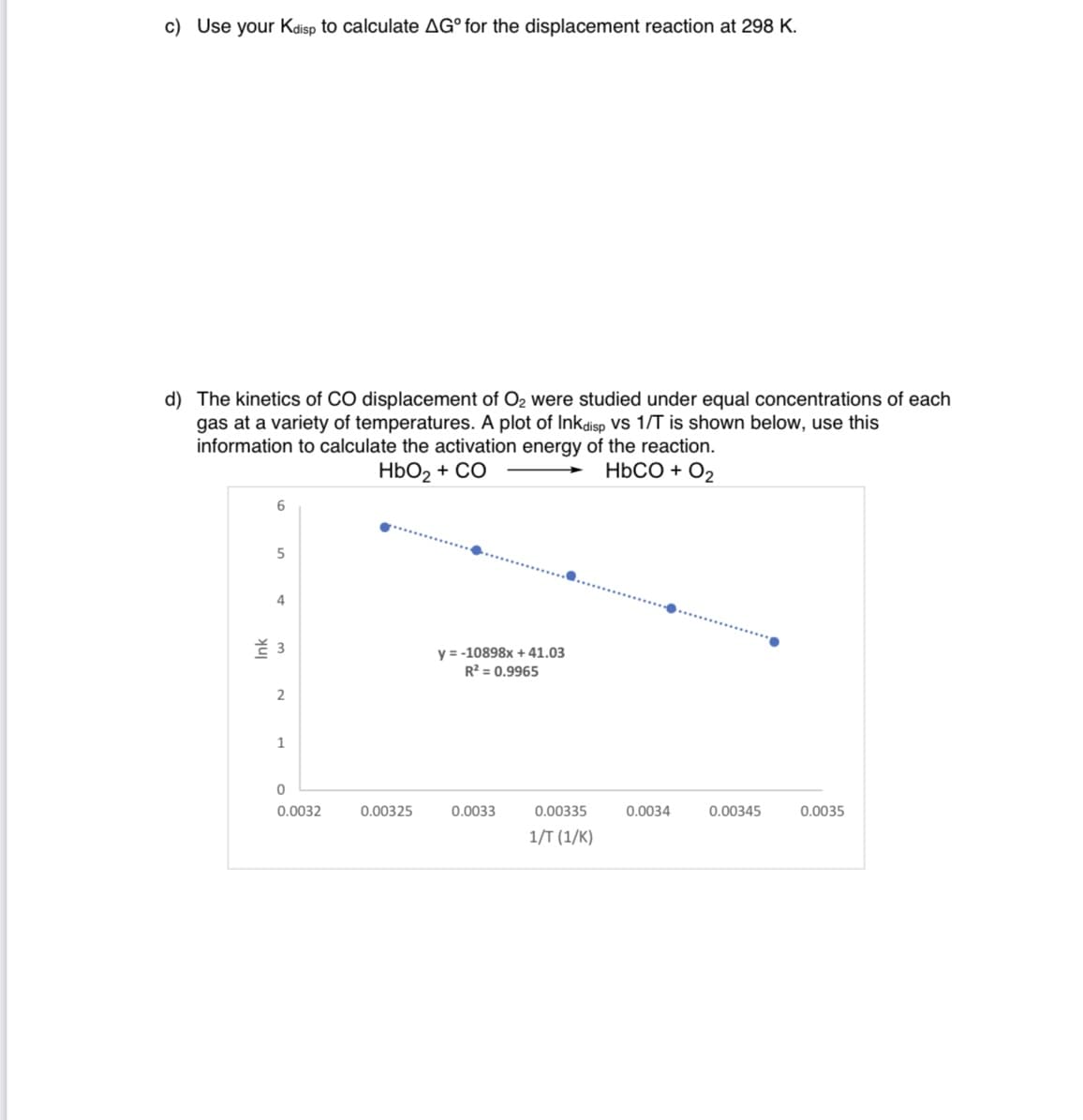
d) The kinetics of CO displacement of O2 were studied under equal concentrations of each gas at a variety of temperatures. A plot of lnkdisp vs 1/T is shown below, use this information to calculate the activation energy of the reaction.
![Hemoglobin (Hb), carries oxygen from our lungs to the rest of out body. The figure below shows
oxygen binding to the active site of Hb.
Fe
N.
Carbon monoxide is poisonous because it binds to Hb in a similar fashion and can displace
oxygen and block O2 from binding to Hb.
a) The reaction below describes the equilibrium for the displacement of O2 in the HbO2
complex by CO to form HBCO. Write an equilibrium expression for this reaction.
HbO2 + CO
НЬСО + О,
Kdisp =
b) The equations below describe the equilibrium for O2 and CO binding to Hb. Using the
information provided determine the value of Kdisp
Hb +O2
HbO2
Ko2 =
[HbO2]
= 3.2
[Hb][02]
Hb + CO
HBCO
[H6C0]
[Hb][CO]
Kco =
= 750](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F02e2307c-cbea-473e-a1a9-a5dce1adbf26%2Fb8388f56-440d-4e8a-9601-169c2bc72ad4%2Fwkgx7kd_processed.jpeg&w=3840&q=75)
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images









