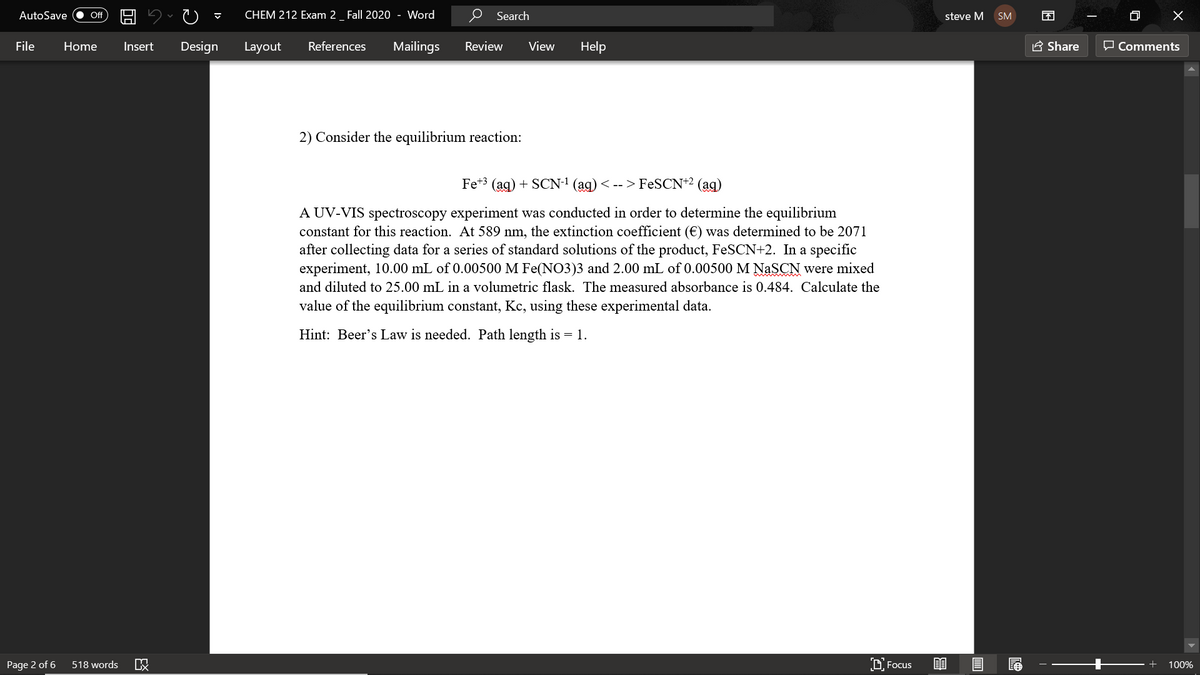
2) Consider the equilibrium reaction: Fe** (ag) + SCN-' (ag) < -- > FeSCN*2 (ag) A UV-VIS spectroscopy experiment was conducted in order to determine the equilibrium constant for this reaction. At 589 nm, the extinction coefficient (€) was determined to be 2071 after collecting data for a series of standard solutions of the product, FeSCN+2. In a specific experiment, 10.00 mL of 0.00500 M Fe(NO3)3 and 2.00 mL of 0.00500 M NASCN were mixed and diluted to 25.00 mL in a volumetric flask. The measured absorbance is 0.484. Calculate the value of the equilibrium constant, Kc, using these experimental data. Hint: Beer's Law is needed. Path length is = 1.
2) Consider the equilibrium reaction: Fe** (ag) + SCN-' (ag) < -- > FeSCN*2 (ag) A UV-VIS spectroscopy experiment was conducted in order to determine the equilibrium constant for this reaction. At 589 nm, the extinction coefficient (€) was determined to be 2071 after collecting data for a series of standard solutions of the product, FeSCN+2. In a specific experiment, 10.00 mL of 0.00500 M Fe(NO3)3 and 2.00 mL of 0.00500 M NASCN were mixed and diluted to 25.00 mL in a volumetric flask. The measured absorbance is 0.484. Calculate the value of the equilibrium constant, Kc, using these experimental data. Hint: Beer's Law is needed. Path length is = 1.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 37P
Related questions
Question
Transcribed Image Text:AutoSave
CHEM 212 Exam 2_ Fall 2020 - Word
O Search
困
ff
steve M
SM
File
Home
Insert
Design
Layout
References
Mailings
Review
View
Help
A Share
O Comments
2) Consider the equilibrium reaction:
Fet3 (ag) + SCN-' (ag) < -- > FESCN+2 (ag)
A UV-VIS spectroscopy experiment was conducted in order to determine the equilibrium
constant for this reaction. At 589 nm, the extinction coefficient (€) was determined to be 2071
after collecting data for a series of standard solutions of the product, FeSCN+2. In a specific
experiment, 10.00 mL of 0.00500 M Fe(NO3)3 and 2.00 mL of 0.00500 M NASCN were mixed
and diluted to 25.00 mL in a volumetric flask. The measured absorbance is 0.484. Calculate the
value of the equilibrium constant, Kc, using these experimental data.
Hint: Beer's Law is needed. Path length is = 1.
Page 2 of 6
518 words
D Focus
100%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning