Carbonyl stretching in transition metal complexes typically occurs in the region 1700 - 2100 cm. How many bands would you expect for the meridional and facial isomers of an WL3(CO)3 complex {where L = P(OCH3)3. structures below) in this region of the spectrum? %3D CO L.. CO L.. co W. W. OC L CO ČO Mer-Complex Fac-Conmplex The compound WL3(CO)3, whose IR spectrum exhibits bands at 1993, 1919 and 1890 cm. What would be the structure of this complex based on the above assignment
Carbonyl stretching in transition metal complexes typically occurs in the region 1700 - 2100 cm. How many bands would you expect for the meridional and facial isomers of an WL3(CO)3 complex {where L = P(OCH3)3. structures below) in this region of the spectrum? %3D CO L.. CO L.. co W. W. OC L CO ČO Mer-Complex Fac-Conmplex The compound WL3(CO)3, whose IR spectrum exhibits bands at 1993, 1919 and 1890 cm. What would be the structure of this complex based on the above assignment
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.30QAP
Related questions
Question
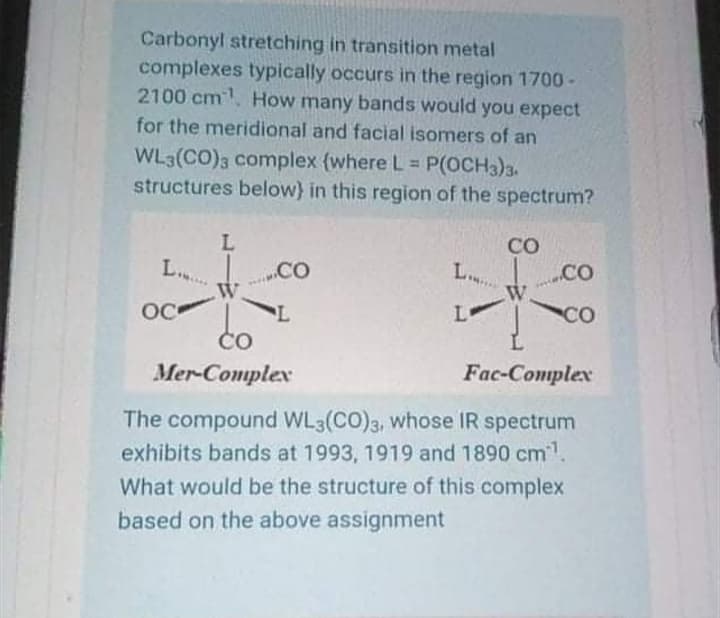
Transcribed Image Text:Carbonyl stretching in transition metal
complexes typically occurs in the region 1700 -
2100 cm. How many bands would you expect
for the meridional and facial isomers of an
WL3(CO)3 complex {where L P(OCH3)3.
structures below} in this region of the spectrum?
CO
L..
.CO
L..
OC
Lo
CO
Čo
Mer-Complex
Fac-Conplex
The compound WL3(CO)3, whose IR spectrum
exhibits bands at 1993, 1919 and 1890 cm1.
What would be the structure of this complex
based on the above assignment
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning