Vanadium forms a complex with peroxide which absorbs at 460 nm. A 3.96 × 10 -4M solution of vanadium peroxide (Solutic A) exhibited an absorbance of 0.624 at 460 nm in a 1.000-cm quartz cuvette; a blank solution containing only solvent had an absorbance of 0.029 at the same wavelength. a) Find the molar absorptivity of vanadium peroxide complex. b) An unknown solution of vanadium peroxide complex in the same solvent and cuvette had an absorbance of 0.375 at 46 nm. Find the concentration of vanadium peroxide in the unknown solution. c) A concentrated solution vanadium peroxide in the same solvent was diluted from an initial volume of 5.00 mL to a final volume of 25.00 mL (Solution C) had an absorbance of 0.733 in the same cuvette. What is the concentration of vanadium peroxide in the concentrated solution (Solution B)? Answer the following questions: 1. Molar Absorptivity (Mª cm ?) of vanadium peroxide complex from Solution A at 460 nm 2. Concentration of vanadium peroxide in unknown solution in mmole/L 3. Concentration of vanadium peroxide in solution C in mmole/L 4. Concentration of vanadium peroxide in concentrated solution (Solution B) in mmole/L
Vanadium forms a complex with peroxide which absorbs at 460 nm. A 3.96 × 10 -4M solution of vanadium peroxide (Solutic A) exhibited an absorbance of 0.624 at 460 nm in a 1.000-cm quartz cuvette; a blank solution containing only solvent had an absorbance of 0.029 at the same wavelength. a) Find the molar absorptivity of vanadium peroxide complex. b) An unknown solution of vanadium peroxide complex in the same solvent and cuvette had an absorbance of 0.375 at 46 nm. Find the concentration of vanadium peroxide in the unknown solution. c) A concentrated solution vanadium peroxide in the same solvent was diluted from an initial volume of 5.00 mL to a final volume of 25.00 mL (Solution C) had an absorbance of 0.733 in the same cuvette. What is the concentration of vanadium peroxide in the concentrated solution (Solution B)? Answer the following questions: 1. Molar Absorptivity (Mª cm ?) of vanadium peroxide complex from Solution A at 460 nm 2. Concentration of vanadium peroxide in unknown solution in mmole/L 3. Concentration of vanadium peroxide in solution C in mmole/L 4. Concentration of vanadium peroxide in concentrated solution (Solution B) in mmole/L
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.19QAP
Related questions
Question
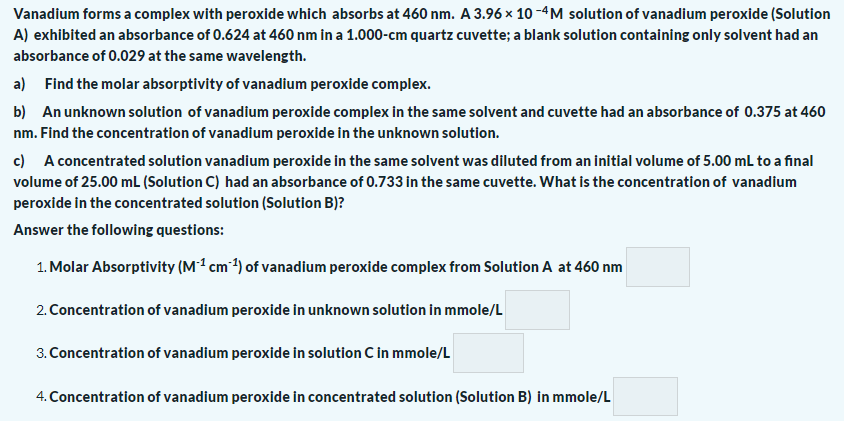
Transcribed Image Text:Vanadium forms a complex with peroxide which absorbs at 460 nm. A 3.96 × 10 -4M solution of vanadium peroxide (Solution
A) exhibited an absorbance of 0.624 at 460 nm in a 1.000-cm quartz cuvette; a blank solution containing only solvent had an
absorbance of 0.029 at the same wavelength.
a) Find the molar absorptivity of vanadium peroxide complex.
b) An unknown solution of vanadium peroxide complex in the same solvent and cuvette had an absorbance of 0.375 at 460
nm. Find the concentration of vanadium peroxide in the unknown solution.
c) A concentrated solution vanadium peroxide in the same solvent was diluted from an initial volume of 5.00 mL to a final
volume of 25.00 mL (Solution C) had an absorbance of 0.733 in the same cuvette. What is the concentration of vanadium
peroxide in the concentrated solution (Solution B)?
Answer the following questions:
1. Molar Absorptivity (M1 cm 1) of vanadium peroxide complex from Solution A at 460 nm
2. Concentration of vanadium peroxide in unknown solution in mmole/L
3. Concentration of vanadium peroxide in solution C in mmole/L
4. Concentration of vanadium peroxide in concentrated solution (Solution B) in mmole/L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning