Chapter30: Orbitals And Organic Chemistry: Pericyclic Reactions
Section30.1: Molecular Orbitals Of Conjugated Pi Systems
Problem 1P
Related questions
Question
By using the given 1) electronic absorption spectrum, 2) the Tanabo-Sugano diagram
for d3 complex in octahedral ligand field and 3) the absorption/
explain the following:
a) Ligand field states (to support your explanation show the filling of the orbitals)
b) Radiative transitions (to support your explanation refer to fluorescence,
phosphorescence and Stokes’ shift)
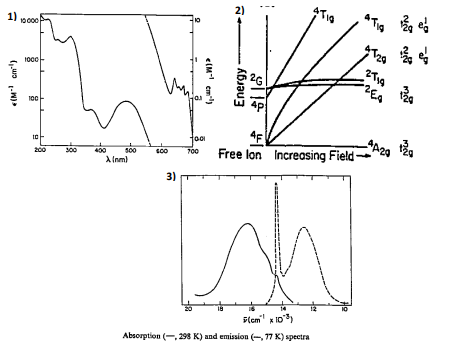
Transcribed Image Text:1) 10000
Mcm)
1000-
100
9
200
300
500
Ainm)
600
3)
elM-1cmly
2)
Energy --
i
2014
4110
700
100 4F
Free lon Increasing Field
Flcm x 103)
Absorption (-,298 K) and emission (-, 77 K) spectra
ATig
2012
12324
210
लूलै রধ
-2E4
2020120
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning