Certain bacteria use the enzyme penicillinase to decom- pose penicillin and render it inactive. The Michaelis- Menten constants for this enzyme and substrate are Km = 5 × 10-5 mol L-ªand k, = 2 × 10³ s¯1. (a) What is the maximum rate of decomposition of peni- cillin if the enzyme concentration is 6 × 10-7 M? (b) At what substrate concentration will the rate of decomposition be half that calculated in part (a)?
Certain bacteria use the enzyme penicillinase to decom- pose penicillin and render it inactive. The Michaelis- Menten constants for this enzyme and substrate are Km = 5 × 10-5 mol L-ªand k, = 2 × 10³ s¯1. (a) What is the maximum rate of decomposition of peni- cillin if the enzyme concentration is 6 × 10-7 M? (b) At what substrate concentration will the rate of decomposition be half that calculated in part (a)?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 116QRT
Related questions
Question
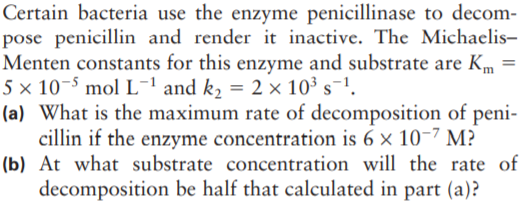
Transcribed Image Text:Certain bacteria use the enzyme penicillinase to decom-
pose penicillin and render it inactive. The Michaelis-
Menten constants for this enzyme and substrate are Km =
5 × 10-5 mol L-ªand k, = 2 × 10³ s¯1.
(a) What is the maximum rate of decomposition of peni-
cillin if the enzyme concentration is 6 × 10-7 M?
(b) At what substrate concentration will the rate of
decomposition be half that calculated in part (a)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning