An atom in the ground state O has all electrons in then 1 orbit O has all electrons in the lowest energy orbits possible O is finely divided O will not absorb electromagnetic radiation of any wavelength Which of the following transitions in the Bohr hydrogen atom model affords emission of the highest-energy photon? (Rh = 2.18 x 10-18 J) O ni = 6 → nf = 3 O ni = 6→ nf = 1 O ni = 1→ nf = 6 O ni = 3 nf = 6 What is the shell electron configuration of halogens? ns?np? or ns? or ns' or ns?np5 O ns1 ns2 ns2np7 ns2np5 The values of the principal quantum number and the angular momentum quantum number of the electrons in a 4d subshell are O 3.3 O 4,3 O 4,2 O 3,2 Which one of the following forms of electromagnetic radiation possesses the greatest energy per photon? O ultraviolet O microwave O X-ray O infrared have the lowest first ionization energies of the groups listed. O Transition elements O Alkaline earth metals Alkali metals O Halogens O Noble gases
An atom in the ground state O has all electrons in then 1 orbit O has all electrons in the lowest energy orbits possible O is finely divided O will not absorb electromagnetic radiation of any wavelength Which of the following transitions in the Bohr hydrogen atom model affords emission of the highest-energy photon? (Rh = 2.18 x 10-18 J) O ni = 6 → nf = 3 O ni = 6→ nf = 1 O ni = 1→ nf = 6 O ni = 3 nf = 6 What is the shell electron configuration of halogens? ns?np? or ns? or ns' or ns?np5 O ns1 ns2 ns2np7 ns2np5 The values of the principal quantum number and the angular momentum quantum number of the electrons in a 4d subshell are O 3.3 O 4,3 O 4,2 O 3,2 Which one of the following forms of electromagnetic radiation possesses the greatest energy per photon? O ultraviolet O microwave O X-ray O infrared have the lowest first ionization energies of the groups listed. O Transition elements O Alkaline earth metals Alkali metals O Halogens O Noble gases
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.71PAE: 6.71 Several excited states of the neon atom are important in the operation of a helium-neon laser....
Related questions
Question
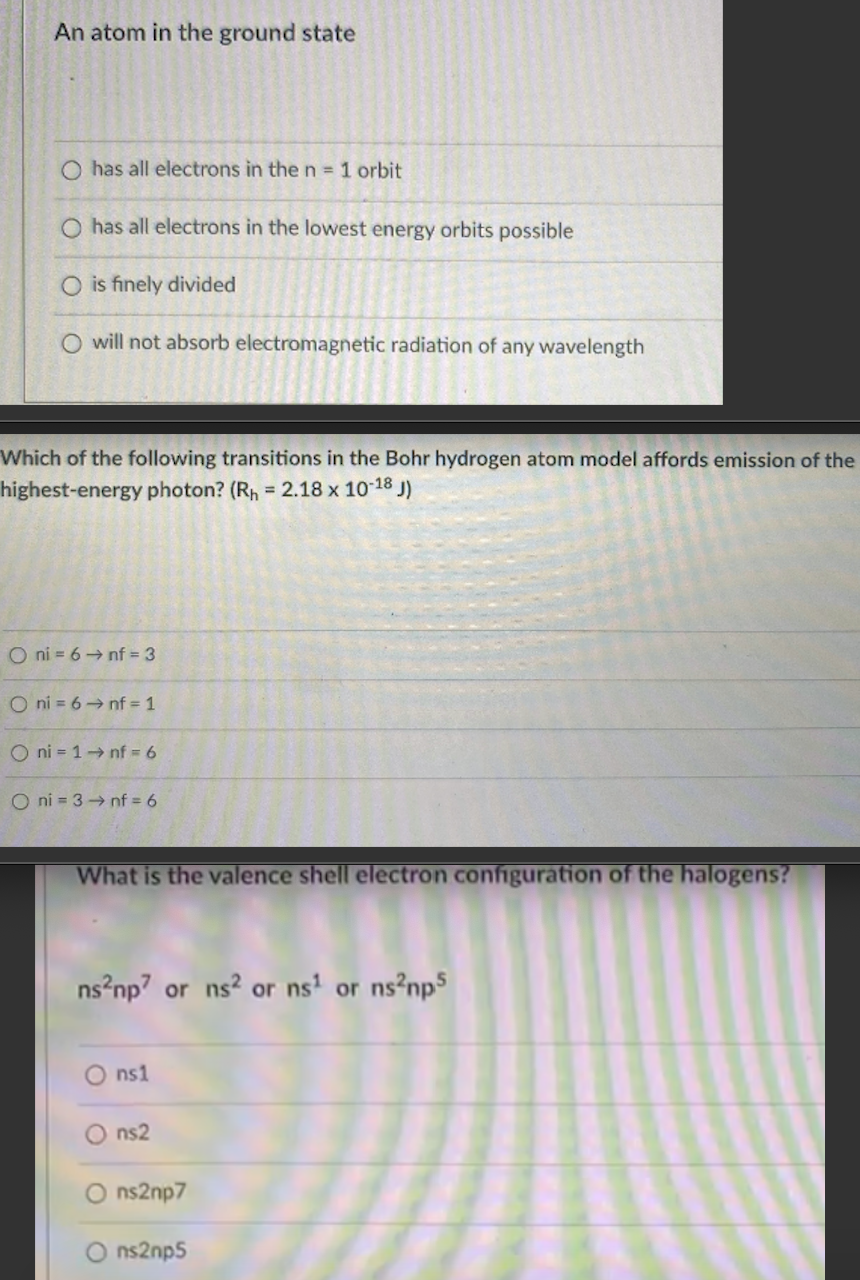
Transcribed Image Text:An atom in the ground state
O has all electrons in then 1 orbit
O has all electrons in the lowest energy orbits possible
O is finely divided
O will not absorb electromagnetic radiation of any wavelength
Which of the following transitions in the Bohr hydrogen atom model affords emission of the
highest-energy photon? (Rh = 2.18 x 10-18 J)
O ni = 6 → nf = 3
O ni = 6→ nf = 1
O ni = 1→ nf = 6
O ni = 3 nf = 6
What is the
shell electron configuration of
halogens?
ns?np? or ns? or ns' or ns?np5
O ns1
ns2
ns2np7
ns2np5
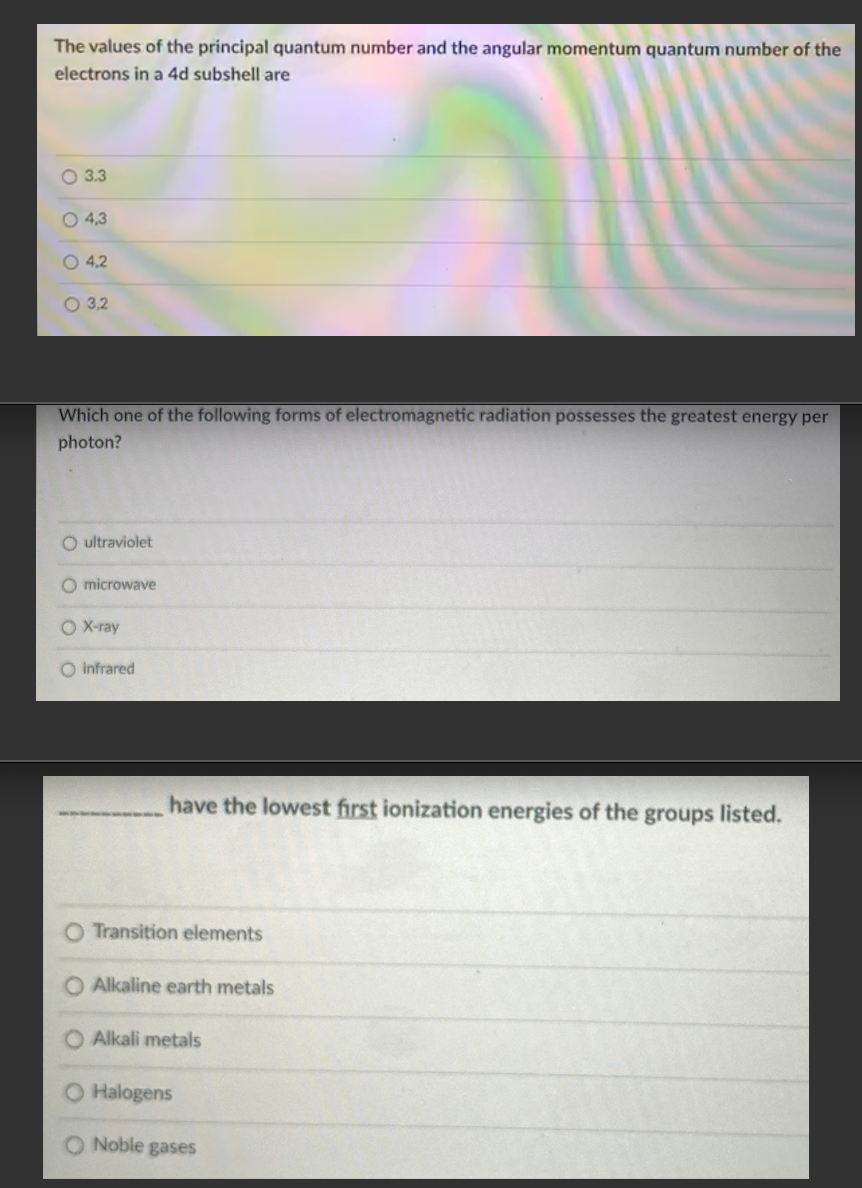
Transcribed Image Text:The values of the principal quantum number and the angular momentum quantum number of the
electrons in a 4d subshell are
O 3.3
O 4,3
O 4,2
O 3,2
Which one of the following forms of electromagnetic radiation possesses the greatest energy per
photon?
O ultraviolet
O microwave
O X-ray
O infrared
have the lowest first ionization energies of the groups listed.
O Transition elements
O Alkaline earth metals
Alkali metals
O Halogens
O Noble gases
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning