Prioritize the substituents on the chiral carbon noted. Assign the highest priority as “1.”
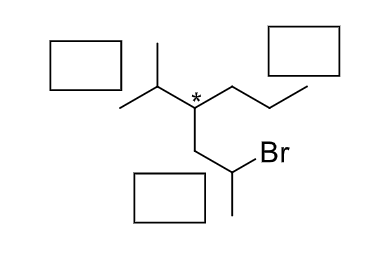
Atomic number depends on priority that means both are direactly proportion to each other.
If atomic number is high then it's priority should be highest and if atomic number is less then it's preority is less.
Priority also depends on 1°, 2° and 3° carbon.
Order of preority, 3° > 2° > 1° carbon this is the decreasing order of priority.
Here you can see that at * carbon one 2° and other two are 2° carbon attached with (star) * carbon so 2° carbon have highest perioriy denoted by 1 and third perioriy is for long chain hydrocarbon ( without Br atom ) because it Br attached with hydrocarbon then it's preority more so that why second perioriy is inwhich Br group attach with long chain carbon.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









