10) Iron has three stable isotopes: 55Fe, 56FE, and 57Fe. The masses and natural abundance of each isotope are listed below. Isotope Isotopic Mass (amu) Natural Abundance (%) 55Fe 54.99 1.79 56FE 55.99 60.30 57Fe 56.99 a. What is the natural abundance of 57Fe? b. What is the atomic mass of iron? You might get a different answer than what is listed on the periodic table. c. If a sample of iron contains 0.582 kg, how many atoms are present? Use the atomic mass you calculated in part (b).
10) Iron has three stable isotopes: 55Fe, 56FE, and 57Fe. The masses and natural abundance of each isotope are listed below. Isotope Isotopic Mass (amu) Natural Abundance (%) 55Fe 54.99 1.79 56FE 55.99 60.30 57Fe 56.99 a. What is the natural abundance of 57Fe? b. What is the atomic mass of iron? You might get a different answer than what is listed on the periodic table. c. If a sample of iron contains 0.582 kg, how many atoms are present? Use the atomic mass you calculated in part (b).
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section2.2: Isotopes And Atomic Weight
Problem 2.3CYU: Neon has three stable isotopes, one with a small abundance. What are the abundances of the other two...
Related questions
Question
I would like help with this question please. Thank you in advance!
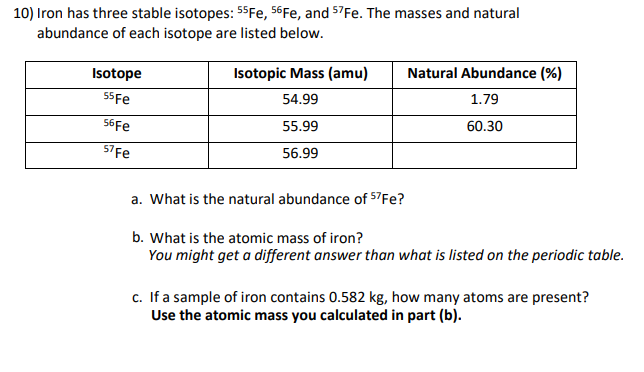
Transcribed Image Text:10) Iron has three stable isotopes: 55Fe, 56FE, and 57Fe. The masses and natural
abundance of each isotope are listed below.
Isotope
Isotopic Mass (amu)
Natural Abundance (%)
55Fe
54.99
1.79
56FE
55.99
60.30
57Fe
56.99
a. What is the natural abundance of 57Fe?
b. What is the atomic mass of iron?
You might get a different answer than what is listed on the periodic table.
c. If a sample of iron contains 0.582 kg, how many atoms are present?
Use the atomic mass you calculated in part (b).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning