01. Suppose you have atoms A and B. Which of the following is not true? A. All matter is composed of small particles called atoms. B. Atoms of one element can be converted to atoms of another element in chemical reactions. C. Atoms have different masses. D. All atoms of a given element are identical. 02. What are isotopes in the given hypothetical elements? Atomic number 53 Mass number Element X Element Y Element Z 54 127 53 131 127 A. X &Z B. X & Y C. Y&Z D. None of the above
01. Suppose you have atoms A and B. Which of the following is not true? A. All matter is composed of small particles called atoms. B. Atoms of one element can be converted to atoms of another element in chemical reactions. C. Atoms have different masses. D. All atoms of a given element are identical. 02. What are isotopes in the given hypothetical elements? Atomic number 53 Mass number Element X Element Y Element Z 54 127 53 131 127 A. X &Z B. X & Y C. Y&Z D. None of the above
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 1E: In the following drawing, the green spheres represent atoms of a certain element. The purple spheres...
Related questions
Question
please answer all
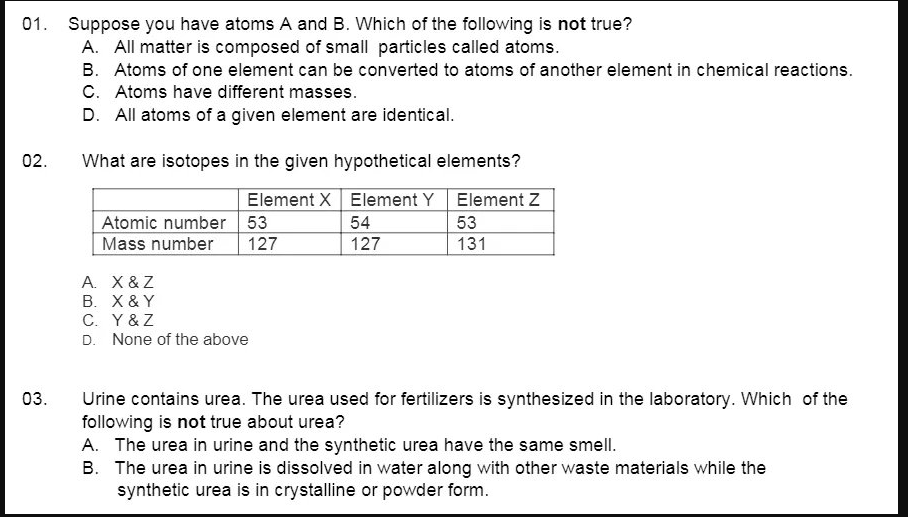
Transcribed Image Text:01. Suppose you have atoms A and B. Which of the following is not true?
A. All matter is composed of small particles called atoms.
B. Atoms of one element can be converted to atoms of another element in chemical reactions.
C. Atoms have different masses.
D. All atoms of a given element are identical.
02.
What are isotopes in the given hypothetical elements?
Element X Element Y Element Z
Atomic number 53
Mass number
54
53
127
127
131
A. X &Z
B. X & Y
C. Y&Z
D. None of the above
03.
Urine contains urea. The urea used for fertilizers is synthesized in the laboratory. Which of the
following is not true about urea?
A. The urea in urine and the synthetic urea have the same smell.
B. The urea in urine is dissolved in water along with other waste materials while the
synthetic urea is in crystalline or powder form.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
