a) Consider the sparingly soluble salt lead(II) sulfate. Write a balanced chemical equation showing the dissolution of lead(II) sulfate. b) Given the following information, provide the net reaction for the spontaneous reaction and determine the cell potential unde standard conditions that would occur from these two half- reactions: Ered Pb2*(aq) + 2e- → Pb(s) 0.1262 V PBSO«(s) + 2e- Pb(s) + SO,2 (aq) Ered 0.3505 V
a) Consider the sparingly soluble salt lead(II) sulfate. Write a balanced chemical equation showing the dissolution of lead(II) sulfate. b) Given the following information, provide the net reaction for the spontaneous reaction and determine the cell potential unde standard conditions that would occur from these two half- reactions: Ered Pb2*(aq) + 2e- → Pb(s) 0.1262 V PBSO«(s) + 2e- Pb(s) + SO,2 (aq) Ered 0.3505 V
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.44QE: For each of the reactions, calculate E from the table of standard potentials, and state whether the...
Related questions
Question
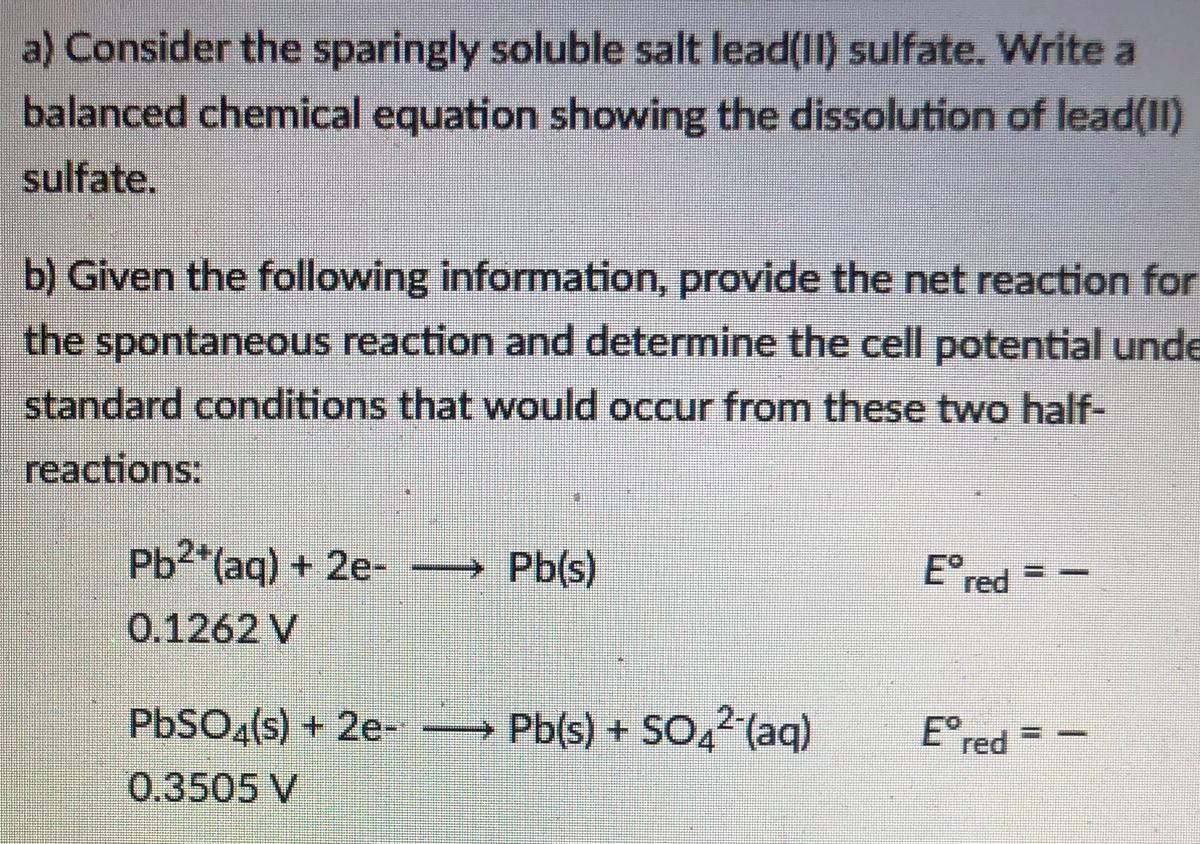
Transcribed Image Text:a) Consider the sparingly soluble salt lead(II) sulfate. Write a
balanced chemical equation showing the dissolution of lead(II)
sulfate.
b) Given the following information, provide the net reaction for
the spontaneous reaction and determine the cell potential unde
standard conditions that would occur from these two half-
reactions:
Ered
Pb2*(aq) + 2e-
→ Pb(s)
0.1262 V
PBSO«(s) + 2e-
Pb(s) + SO,2 (aq)
Ered
0.3505 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning