Question 4 Which of these substances are compounds? L. Neon II. Crude oil III. Water IV. Sodium chloride o I and III o II, III, and IV O III and IV O Il and IV Question 5 Which scientist is incorrectly matched with his idea or theory? Scientist Theory I. Rutherford Atoms contain a nucleus. IL Proust Law of constant composition III. Lavoisier Law of conservation of matter IV. Dalton Law of conservation of mass V. Boyle The simplest form of a substance is an element. O I. O III. O IV. O V. Question 6 Methane can be decomposed into two simpler substances: hydrogen and carbon. Therefore, methane is a(n) a mixture O clement atom compound. O molecule
Question 4 Which of these substances are compounds? L. Neon II. Crude oil III. Water IV. Sodium chloride o I and III o II, III, and IV O III and IV O Il and IV Question 5 Which scientist is incorrectly matched with his idea or theory? Scientist Theory I. Rutherford Atoms contain a nucleus. IL Proust Law of constant composition III. Lavoisier Law of conservation of matter IV. Dalton Law of conservation of mass V. Boyle The simplest form of a substance is an element. O I. O III. O IV. O V. Question 6 Methane can be decomposed into two simpler substances: hydrogen and carbon. Therefore, methane is a(n) a mixture O clement atom compound. O molecule
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 166SCQ: A jar contains some number of jelly beans. To find out precisely how many are in the jar, you could...
Related questions
Question
Hi,
I am doing a practice test and need to check my answers. All of this is for Chem 101
Please see attached:
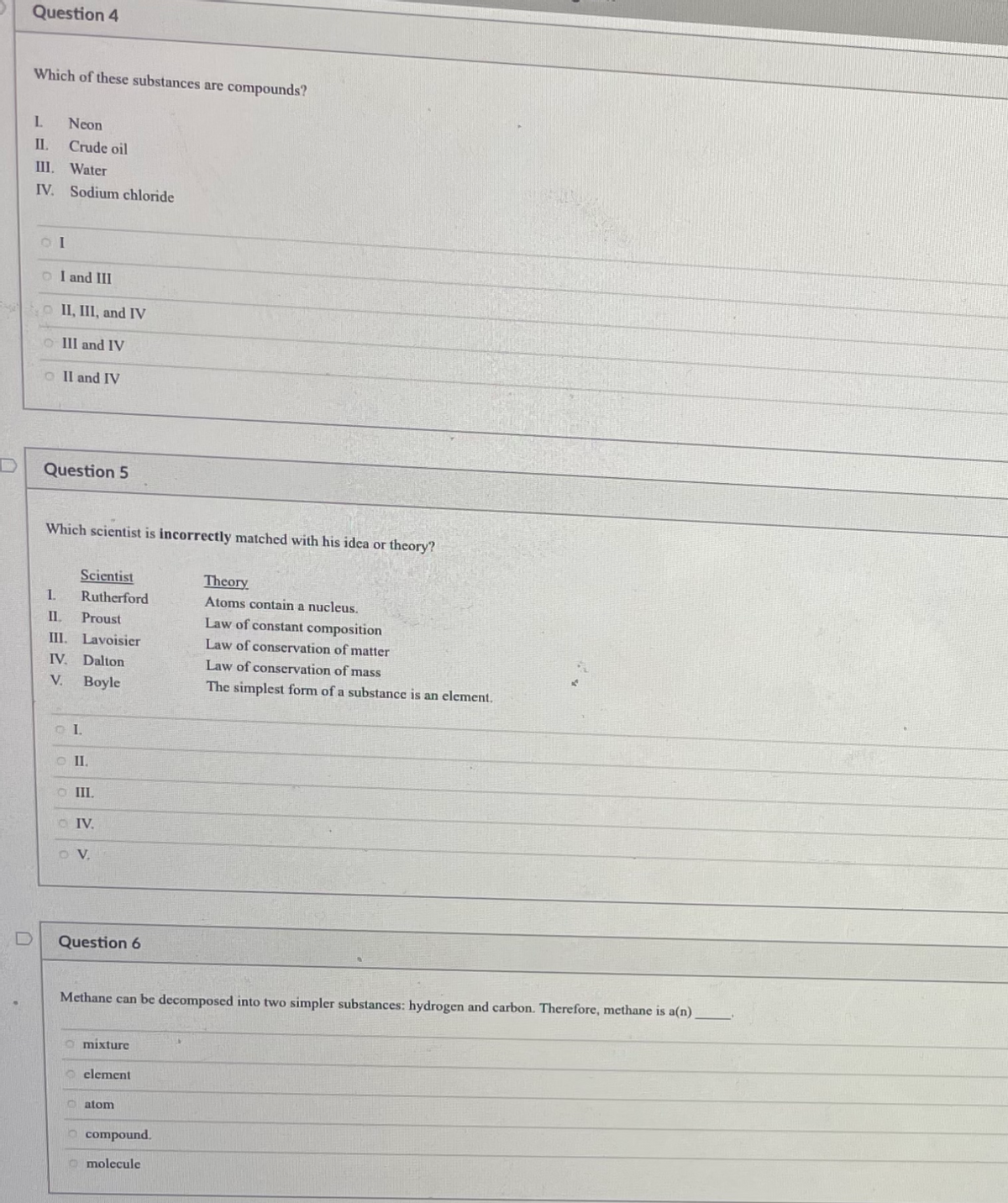
Transcribed Image Text:Question 4
Which of these substances are compounds?
L.
Neon
II.
Crude oil
III. Water
IV. Sodium chloride
o I and III
o II, III, and IV
O III and IV
O Il and IV
Question 5
Which scientist is incorrectly matched with his idea or theory?
Scientist
Theory
I.
Rutherford
Atoms contain a nucleus.
IL
Proust
Law of constant composition
III. Lavoisier
Law of conservation of matter
IV. Dalton
Law of conservation of mass
V.
Boyle
The simplest form of a substance is an element.
O I.
O III.
O IV.
O V.
Question 6
Methane can be decomposed into two simpler substances: hydrogen and carbon. Therefore, methane is a(n)
a mixture
O clement
atom
compound.
O molecule
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning