1. (a) Explain why compound A is optically active while compound B is optically inactive at room temperature. •. .. P. PHCH2 H. Me Me А В (b) If optically active compound A is dissolved in water containing a small amount of H3O*, its optical rotation rapidly decreases in magnitude, eventually becoming 0. Provide an explanation for this observation, using structures and chemical reactions.
1. (a) Explain why compound A is optically active while compound B is optically inactive at room temperature. •. .. P. PHCH2 H. Me Me А В (b) If optically active compound A is dissolved in water containing a small amount of H3O*, its optical rotation rapidly decreases in magnitude, eventually becoming 0. Provide an explanation for this observation, using structures and chemical reactions.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.58P
Related questions
Question
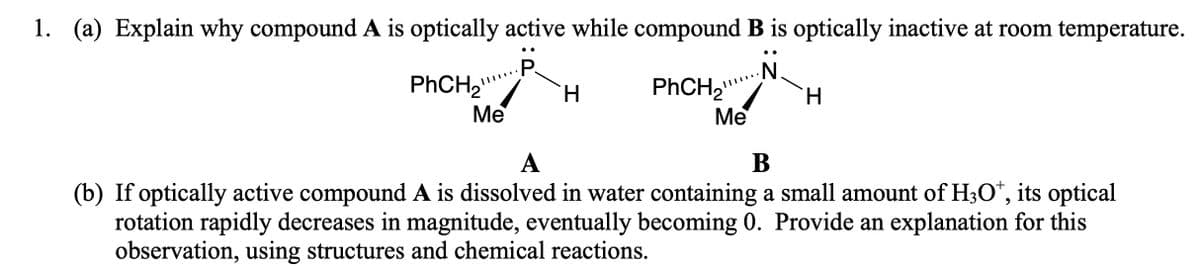
Transcribed Image Text:1. (a) Explain why compound A is optically active while compound B is optically inactive at room temperature.
•.
..
P.
PHCH2
H.
Me
Me
А
В
(b) If optically active compound A is dissolved in water containing a small amount of H3O*, its optical
rotation rapidly decreases in magnitude, eventually becoming 0. Provide an explanation for this
observation, using structures and chemical reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning