O Emmaree Hernandez - Mole R X How to Calculate Molar Mass O Unit 4 Test Choice Boarc X A IN CLASS ASSIGNMENT FOR 1 X A goformative.com/formatives/6021509f9fbcc1919474d934 4 7. 8 9 10 5.717 grams H2 9 In the reaction: N, + 3H, 2NH, what is the mole ratio of hydrogen to nitrogen? 3. O3:2 1:1 1:2 О 3:1
O Emmaree Hernandez - Mole R X How to Calculate Molar Mass O Unit 4 Test Choice Boarc X A IN CLASS ASSIGNMENT FOR 1 X A goformative.com/formatives/6021509f9fbcc1919474d934 4 7. 8 9 10 5.717 grams H2 9 In the reaction: N, + 3H, 2NH, what is the mole ratio of hydrogen to nitrogen? 3. O3:2 1:1 1:2 О 3:1
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 133CP
Related questions
Question
100%
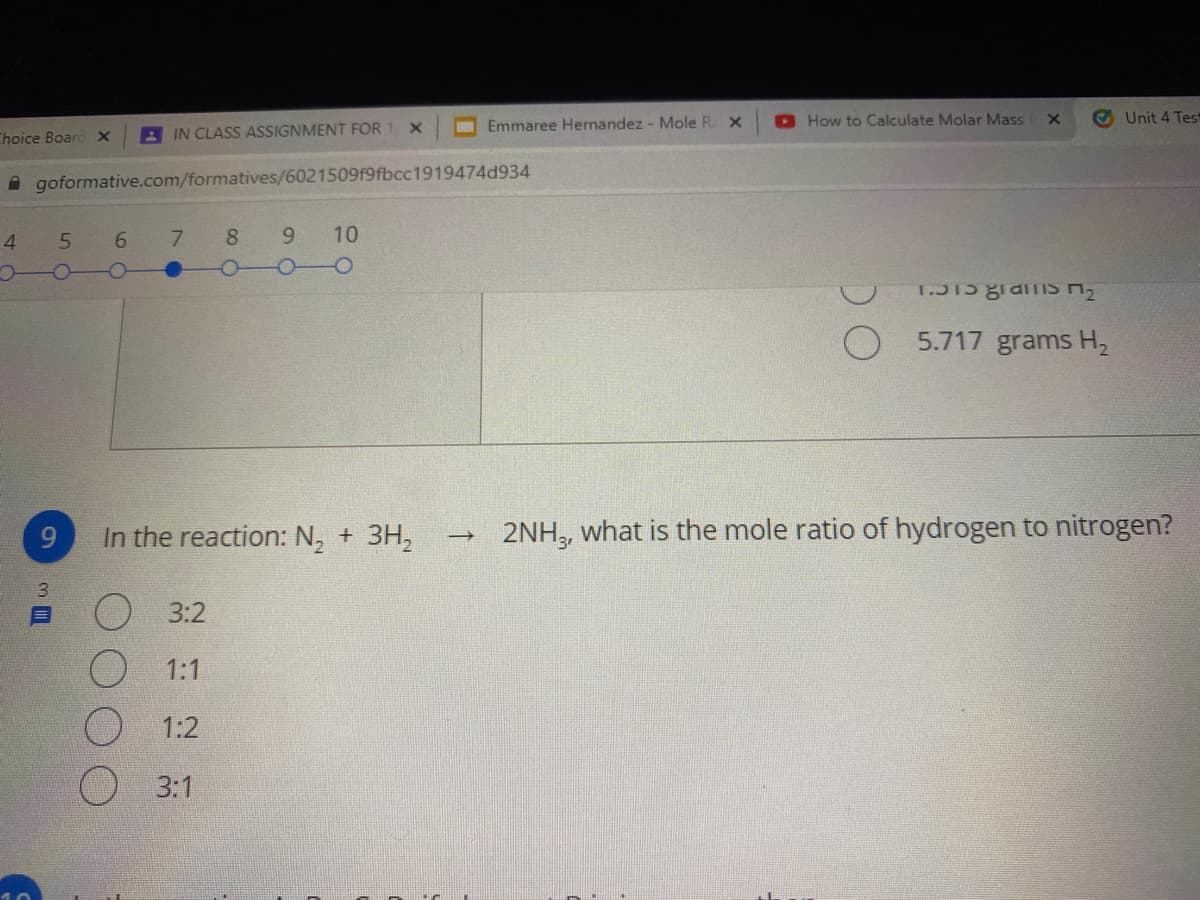
Transcribed Image Text:O Emmaree Hernandez - Mole R X
How to Calculate Molar Mass
O Unit 4 Test
Choice Boarc X
A IN CLASS ASSIGNMENT FOR 1 X
A goformative.com/formatives/6021509f9fbcc1919474d934
4
7.
8 9
10
5.717 grams H2
9
In the reaction: N, + 3H,
2NH, what is the mole ratio of hydrogen to nitrogen?
3.
O3:2
1:1
1:2
О 3:1
Expert Solution
Step 1
The given reaction is :
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co