Chemists in the 19th century constructed an evacuated glass tube with wires inserted in both ends. When an electrical current was generated at the anode, a 'cathode ray' would pass to the cathode creating a fluorescent glow. These experiments helped chemists better understand the nature of the electron. J.J. Thomson added a magnetic field to a cathode ray tube to show that the ray would bend. His experiments demonstrated that the rays were negatively charged particles. He then calculated the mass to charge ratio of the electric ion (electron). According to his calculations, how massive was the electron relative to the hydrogen ion? Voltage Source A) The same size B) -1000 times larger C) ~1000 times smaller D) -10 times larger Anode Cathode E) ~10 times smaller
Chemists in the 19th century constructed an evacuated glass tube with wires inserted in both ends. When an electrical current was generated at the anode, a 'cathode ray' would pass to the cathode creating a fluorescent glow. These experiments helped chemists better understand the nature of the electron. J.J. Thomson added a magnetic field to a cathode ray tube to show that the ray would bend. His experiments demonstrated that the rays were negatively charged particles. He then calculated the mass to charge ratio of the electric ion (electron). According to his calculations, how massive was the electron relative to the hydrogen ion? Voltage Source A) The same size B) -1000 times larger C) ~1000 times smaller D) -10 times larger Anode Cathode E) ~10 times smaller
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 7P
Related questions
Question
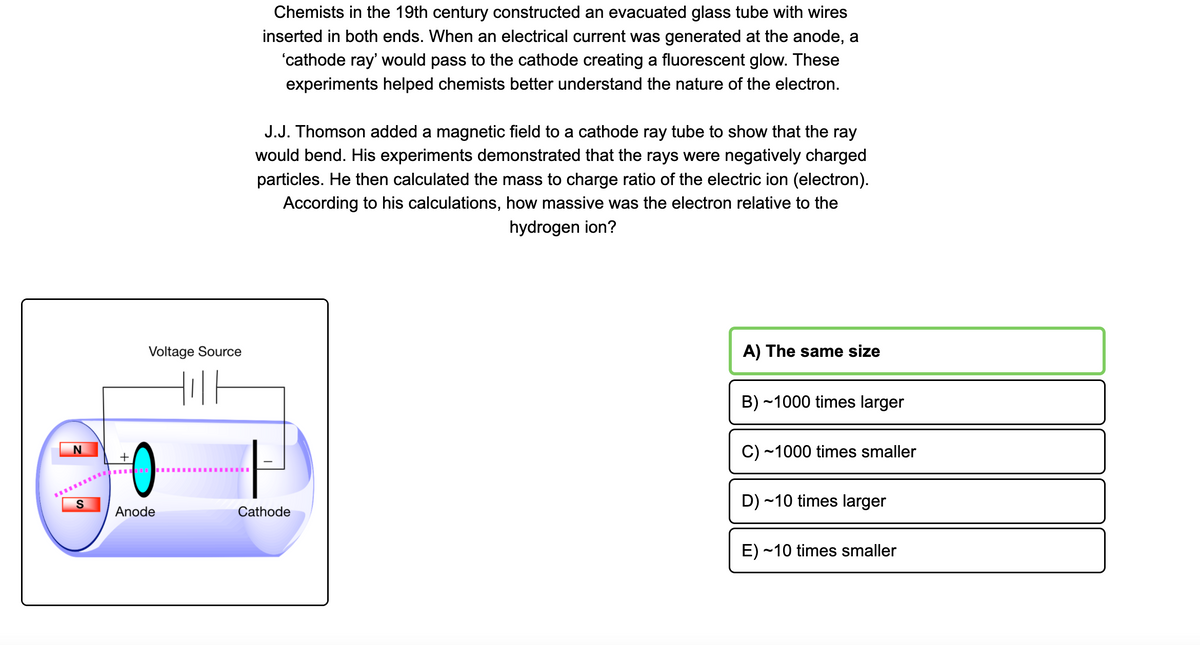
Transcribed Image Text:Chemists in the 19th century constructed an evacuated glass tube with wires
inserted in both ends. When an electrical current was generated at the anode, a
'cathode ray' would pass to the cathode creating a fluorescent glow. These
experiments helped chemists better understand the nature of the electron.
J.J. Thomson added a magnetic field to a cathode ray tube to show that the ray
would bend. His experiments demonstrated that the rays were negatively charged
particles. He then calculated the mass to charge ratio of the electric ion (electron).
According to his calculations, how massive was the electron relative to the
hydrogen ion?
Voltage Source
A) The same size
B) ~1000 times larger
C) ~1000 times smaller
+
S
D) ~10 times larger
Anode
Cathode
E) ~10 times smaller
Expert Solution
Step 1
Chemists in the 19th century constructed an evacuated glass tube with wires inserted in both ends. When an electrical current was generated at the anode, a 'cathode ray' would pass to the cathode creating a fluorescent glow.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning