The color of dyes results from the preferential absorption of certain wavelengths of light. Certain dye molecules consist of symmetric pairs of rings joined at the center by a chain of carbon atoms, as shown in (Figure 1). Electrons of the bonds along the chain of carbon atoms are shared among the atoms in the chain, but are repelled by the nitrogen- containing rings at the end of the chain. These electrons are thus free to move along the chain but not beyond its ends. They look very much like a particle in a one-dimensional box. For the molecule shown, the effective length of the "box" is 0.85 nm. Part A Assuming that the electrons start in the lowest energy state, what is the longest wavelength this molecule will absorb? Express your answer with the appropriate units. HA ? Value Units Submit Request Answer
The color of dyes results from the preferential absorption of certain wavelengths of light. Certain dye molecules consist of symmetric pairs of rings joined at the center by a chain of carbon atoms, as shown in (Figure 1). Electrons of the bonds along the chain of carbon atoms are shared among the atoms in the chain, but are repelled by the nitrogen- containing rings at the end of the chain. These electrons are thus free to move along the chain but not beyond its ends. They look very much like a particle in a one-dimensional box. For the molecule shown, the effective length of the "box" is 0.85 nm. Part A Assuming that the electrons start in the lowest energy state, what is the longest wavelength this molecule will absorb? Express your answer with the appropriate units. HA ? Value Units Submit Request Answer
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 87AP: At large interatomic separations, an alkali halide molecule MX has a lower energy as two neutral...
Related questions
Question
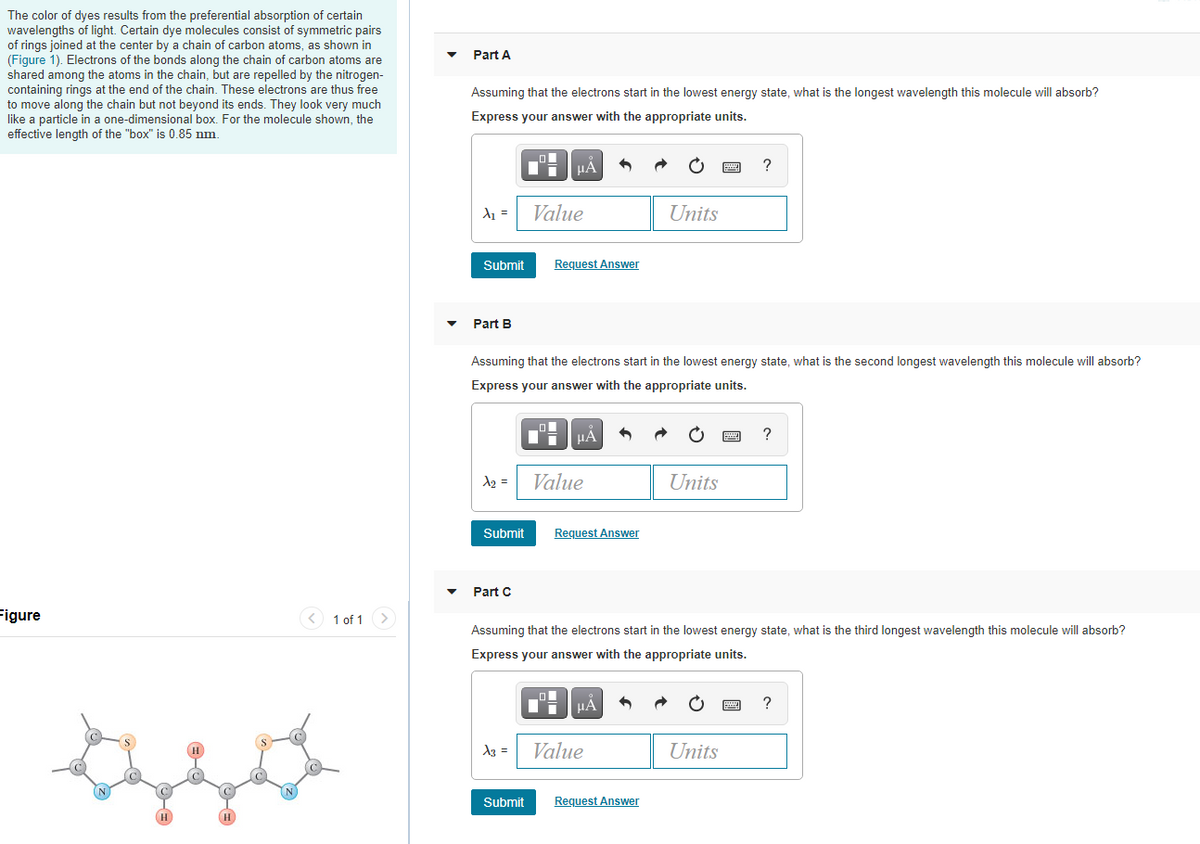
Transcribed Image Text:The color of dyes results from the preferential absorption of certain
wavelengths of light. Certain dye molecules consist of symmetric pairs
of rings joined at the center by a chain of carbon atoms, as shown in
(Figure 1). Electrons of the bonds along the chain of carbon atoms are
shared among the atoms in the chain, but are repelled by the nitrogen-
containing rings at the end of the chain. These electrons are thus free
to move along the chain but not beyond its ends. They look very much
like a particle in a one-dimensional box. For the molecule shown, the
effective length of the "box" is 0.85 nm.
Part A
Assuming that the electrons start in the lowest energy state, what is the longest wavelength this molecule will absorb?
Express your answer with the appropriate units.
HÀ
?
d1 =
Value
Units
Submit
Request Answer
Part B
Assuming that the electrons start in the lowest energy state, what is the second longest wavelength this molecule will absorb?
Express your answer with the appropriate units.
HA
A2 =
Value
Units
Submit
Request Answer
Part C
Figure
< 1 of 1 >
Assuming that the electrons start in the lowest energy state, what is the third longest wavelength this molecule will absorb?
Express your answer with the appropriate units.
μΑ
A3 =
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co