Choose the statements that describe why the isomerization reaction is critical for the subsequent cleavage reaction that results in formation of two three-carbon sugars in the glycolytic pathway. The carbon of a carbonyl group has a partial positive charge and can delocalize electrons to facilitate cleavage of the C-C bond. Without the isomerization reaction, C-1 will be the carbonyl carbon and the C-C cleavage will occur between C-2 and C-3. Breaking a C-C bond is energetically unfavorable and can only proceed if a functional group with electronegative atoms is adjacent to the cleavage site to stabilize the reaction intermediates. To cleave a C-C bond, a nucleophilic carbon attacks the adjacent electrophilic carbon. The isomerization reaction moves the carbonyl group to C-2, thus making it a nucleophile that can attack C-3 to generate two three-carbon sugars. ☐ The partial positive charge on the carbonyl carbon can stabilize the carbon radicals formed by the homolytic cleavage of the C-C bond in the six-carbon sugar. Since the C-C cleavage occurs between C-3 and C-4 of the six-carbon sugar, the carbonyl group needs to be on C-2. Cleavage of a C-C bond in the six-carbon sugar occurs via a decarboxylation reaction that requires a carbonyl group adjacent to cleavage site to stabilize the carbanion intermediate.
Choose the statements that describe why the isomerization reaction is critical for the subsequent cleavage reaction that results in formation of two three-carbon sugars in the glycolytic pathway. The carbon of a carbonyl group has a partial positive charge and can delocalize electrons to facilitate cleavage of the C-C bond. Without the isomerization reaction, C-1 will be the carbonyl carbon and the C-C cleavage will occur between C-2 and C-3. Breaking a C-C bond is energetically unfavorable and can only proceed if a functional group with electronegative atoms is adjacent to the cleavage site to stabilize the reaction intermediates. To cleave a C-C bond, a nucleophilic carbon attacks the adjacent electrophilic carbon. The isomerization reaction moves the carbonyl group to C-2, thus making it a nucleophile that can attack C-3 to generate two three-carbon sugars. ☐ The partial positive charge on the carbonyl carbon can stabilize the carbon radicals formed by the homolytic cleavage of the C-C bond in the six-carbon sugar. Since the C-C cleavage occurs between C-3 and C-4 of the six-carbon sugar, the carbonyl group needs to be on C-2. Cleavage of a C-C bond in the six-carbon sugar occurs via a decarboxylation reaction that requires a carbonyl group adjacent to cleavage site to stabilize the carbanion intermediate.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 14P: Energetic of Fructose-1 ,6-bis P Hydrolysis (Integrates with Chapter 3.) The standard free energy...
Related questions
Question
Transcribed Image Text:Macmillan Learning
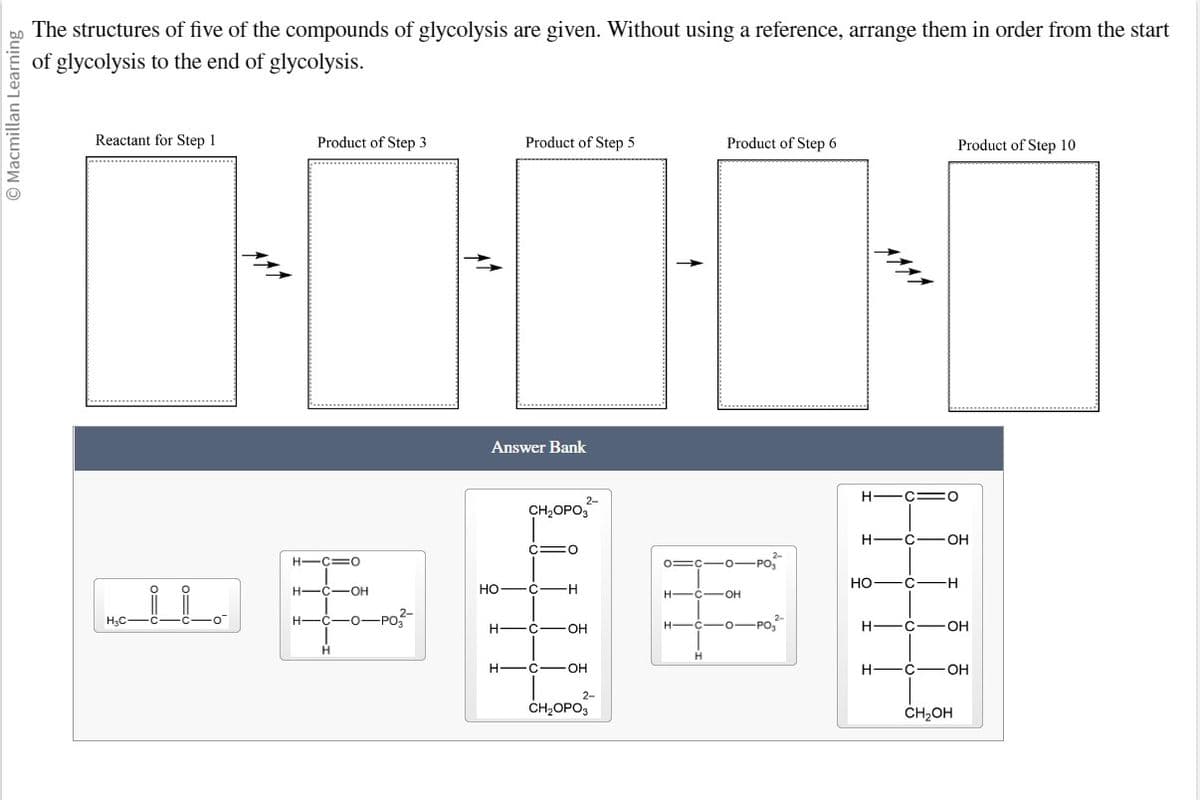
The structures of five of the compounds of glycolysis are given. Without using a reference, arrange them in order from the start
of glycolysis to the end of glycolysis.
Reactant for Step 1
H₂C
Product of Step 3
H-C=O
H-C-OH
H-C- -O-PO
H
Product of Step 5
Answer Bank
HO
CH₂OPO3
CO
2-
C H
HTC OH
H COH
2-
CH₂OPO3
Product of Step 6
0=ç-0-PO,
H-C
OH
HIC-O-PO
H-C=0
H
Product of Step 10
C-OH
но- C-H
H C-OH
H-C-OH
CH₂OH
Transcribed Image Text:Macmillan Learning
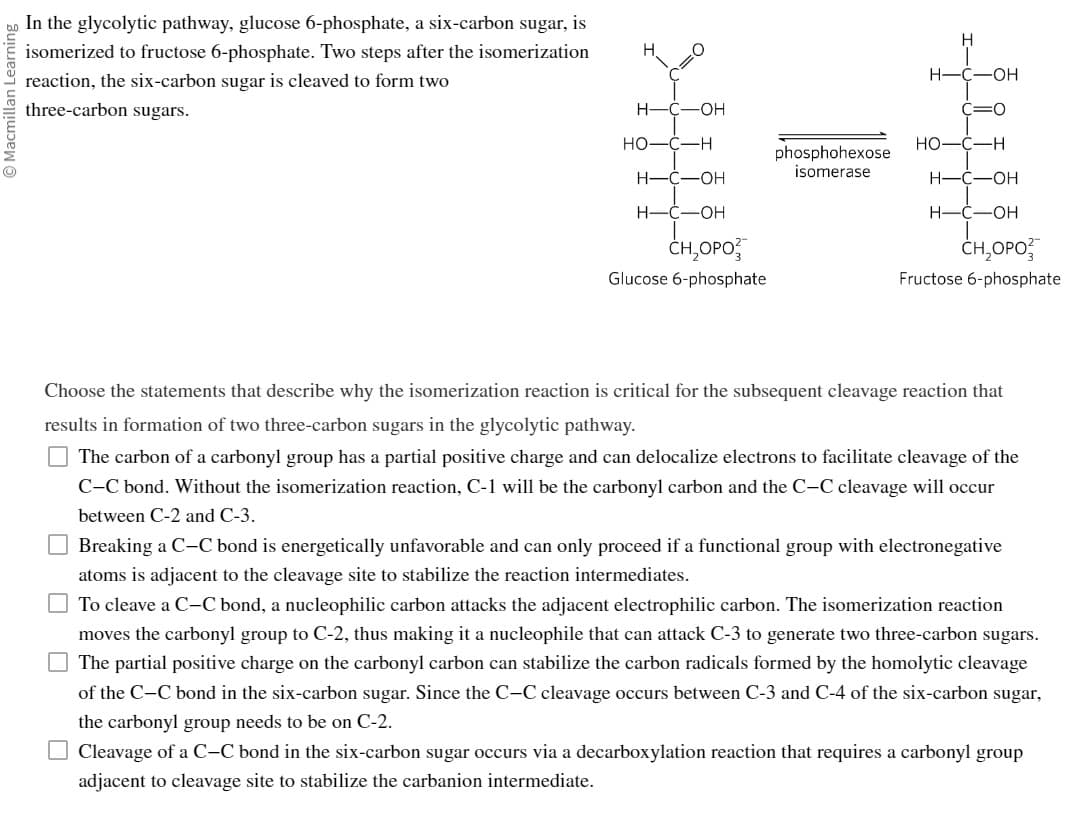
In the glycolytic pathway, glucose 6-phosphate, a six-carbon sugar, is
isomerized to fructose 6-phosphate. Two steps after the isomerization
reaction, the six-carbon sugar is cleaved to form two
three-carbon sugars.
H-
-OH
H
H-C- -OH
HO
H-C-OH
CH₂OPO
Glucose 6-phosphate
phosphohexose
isomerase
H
H-C-OH
O
HO-C-H
H-C-OH
H-C -OH
CH₂OPO
Fructose 6-phosphate
Choose the statements that describe why the isomerization reaction is critical for the subsequent cleavage reaction that
results in formation of two three-carbon sugars in the glycolytic pathway.
The carbon of a carbonyl group has a partial positive charge and can delocalize electrons to facilitate cleavage of the
C-C bond. Without the isomerization reaction, C-1 will be the carbonyl carbon and the C-C cleavage will occur
between C-2 and C-3.
Breaking a C-C bond is energetically unfavorable and can only proceed if a functional group with electronegative
atoms is adjacent to the cleavage site to stabilize the reaction intermediates.
To cleave a C-C bond, a nucleophilic carbon attacks the adjacent electrophilic carbon. The isomerization reaction
moves the carbonyl group to C-2, thus making it a nucleophile that can attack C-3 to generate two three-carbon sugars.
The partial positive charge on the carbonyl carbon can stabilize the carbon radicals formed by the homolytic cleavage
of the C-C bond in the six-carbon sugar. Since the C-C cleavage occurs between C-3 and C-4 of the six-carbon sugar,
the carbonyl group needs to be on C-2.
Cleavage of a C-C bond in the six-carbon sugar occurs via a decarboxylation reaction that requires a carbonyl group
adjacent to cleavage site to stabilize the carbanion intermediate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning