Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
I struggle with this question.

Transcribed Image Text:CONCENTRATION
-
Cin (mM)
Cout (mM)
Na
50.0
440.0
K
400.0
20.0
C
52.0
560.0
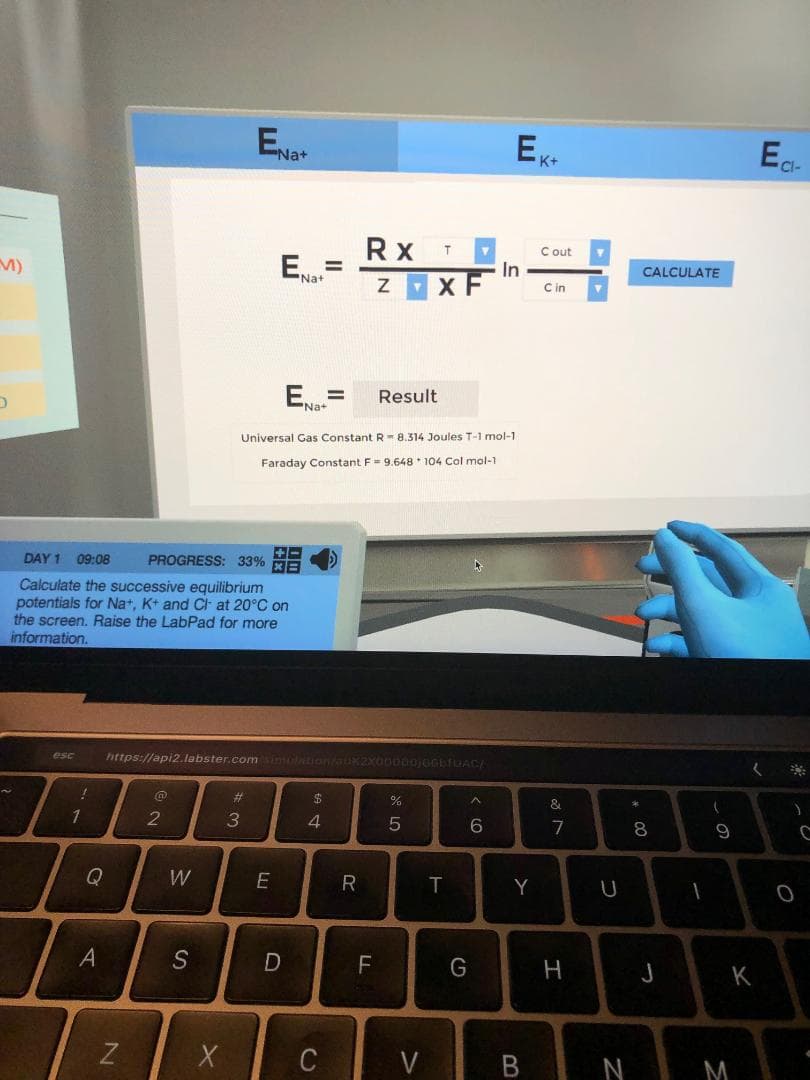
Transcribed Image Text:Ea
Na+
Rx
C out
ENa
In
%3D
CALCULATE
x F
C in
E=
%D
'Na+
Result
Universal Gas Constant R - 8.314 Joules T-1 mol-1
Faraday Constant F- 9.648 * 104 Col mol-1
DAY 1
09:08
PROGRESS: 33% HE
Calculate the successive equilibrium
potentials for Na+, K+ and C- at 20°C on
the screen. Raise the LabPad for more
information.
esc
https://api2.labster.com
23
$
&
1
3
4
8
Q
W
T
Y
A
S
H
K
C
V
B N M
* CC
LLI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning