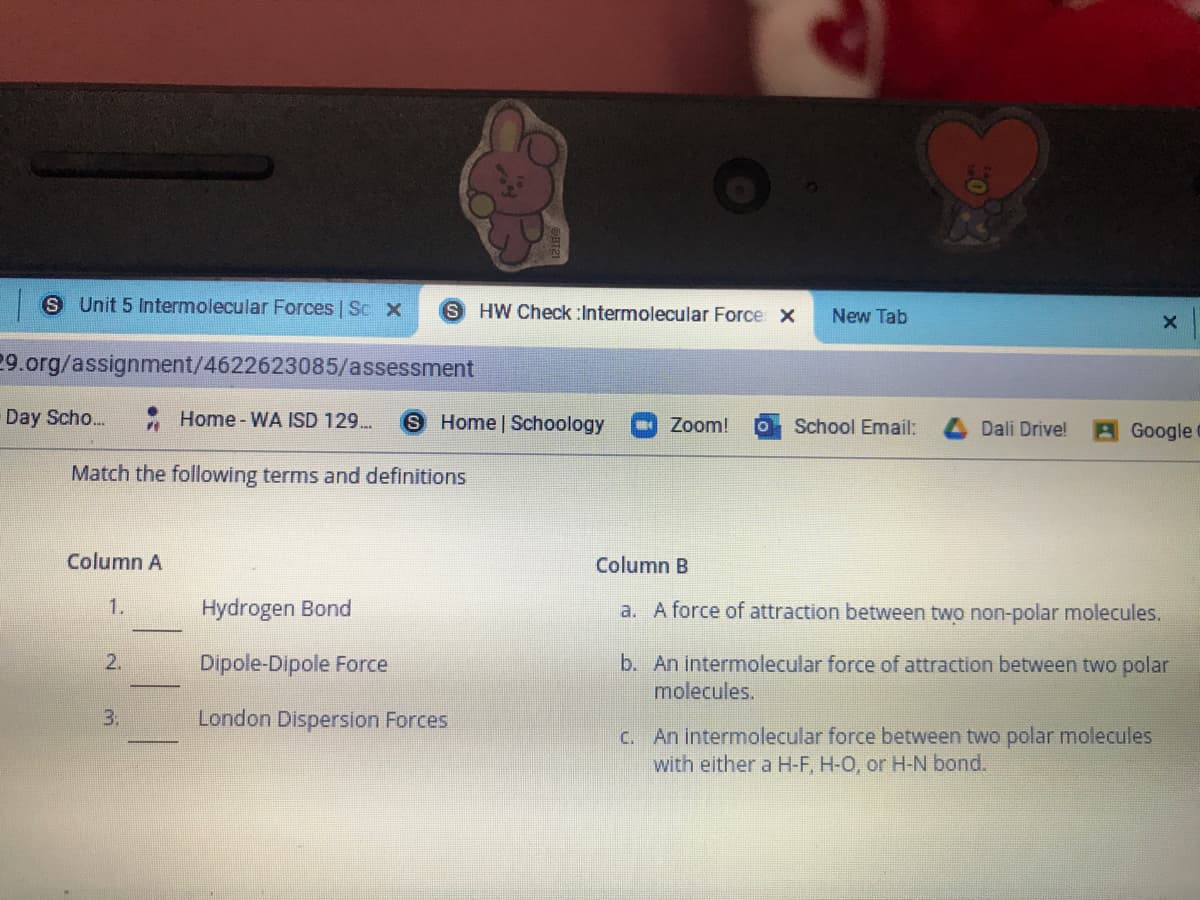
Column A Column B 1. Hydrogen Bond a. A force of attraction between two non-polar molecules. Dipole-Dipole Force b. An intermolecular force of attraction between two polar molecules. 2. 3. London Dispersion Forces C. An intermolecular force between two polar molecules with either a H-F, H-O, or H-N bond.
Column A Column B 1. Hydrogen Bond a. A force of attraction between two non-polar molecules. Dipole-Dipole Force b. An intermolecular force of attraction between two polar molecules. 2. 3. London Dispersion Forces C. An intermolecular force between two polar molecules with either a H-F, H-O, or H-N bond.
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 1RQ: What are intermolecular forces? How do they differ from intramolecular forces? What are...
Related questions
Question
7. I need help
Transcribed Image Text:S Unit 5 Intermolecular Forces | Sc X
S HW Check :Intermolecular Force X
New Tab
29.org/assignment/4622623085/assessment
Day Scho...
Home - WA ISD 129..
S Home Schoology
Zoom!
School Email:
Dali Drive!
Google
Match the following terms and definitions
Column A
Column B
1.
Hydrogen Bond
a. A force of attraction between two non-polar molecules.
2.
Dipole-Dipole Force
b. An intermolecular force of attraction between two polar
molecules.
3.
London Dispersion Forces
C. An intermolecular force between two polar molecules
with either a H-F, H-O, or H-N bond.
Expert Solution
Step 1
Intermolecular forces refer to the type of interactions which are present in between atoms or molecules. These are responsible for holding different molecules together. There are mainly three types which are dispersion forces, dipole-dipole forces and hydrogen bonding.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co