Which of the following statements about hydrogen bonds is NOT correct? OA. Hydrogen bonds act inside each water molecule to attach both hydrogen atoms to a single oxygen atom. O B. Hydrogen bonds form because water has a strong dipole. OC. Hydrogen bonds act as an attractive force between water molecules. OD. Hydrogen bonds form because oxygen draws electron density away from hydrogen in a water molecule. Reset Selection vious NEXT Save Exit
Which of the following statements about hydrogen bonds is NOT correct? OA. Hydrogen bonds act inside each water molecule to attach both hydrogen atoms to a single oxygen atom. O B. Hydrogen bonds form because water has a strong dipole. OC. Hydrogen bonds act as an attractive force between water molecules. OD. Hydrogen bonds form because oxygen draws electron density away from hydrogen in a water molecule. Reset Selection vious NEXT Save Exit
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.107E
Related questions
Question
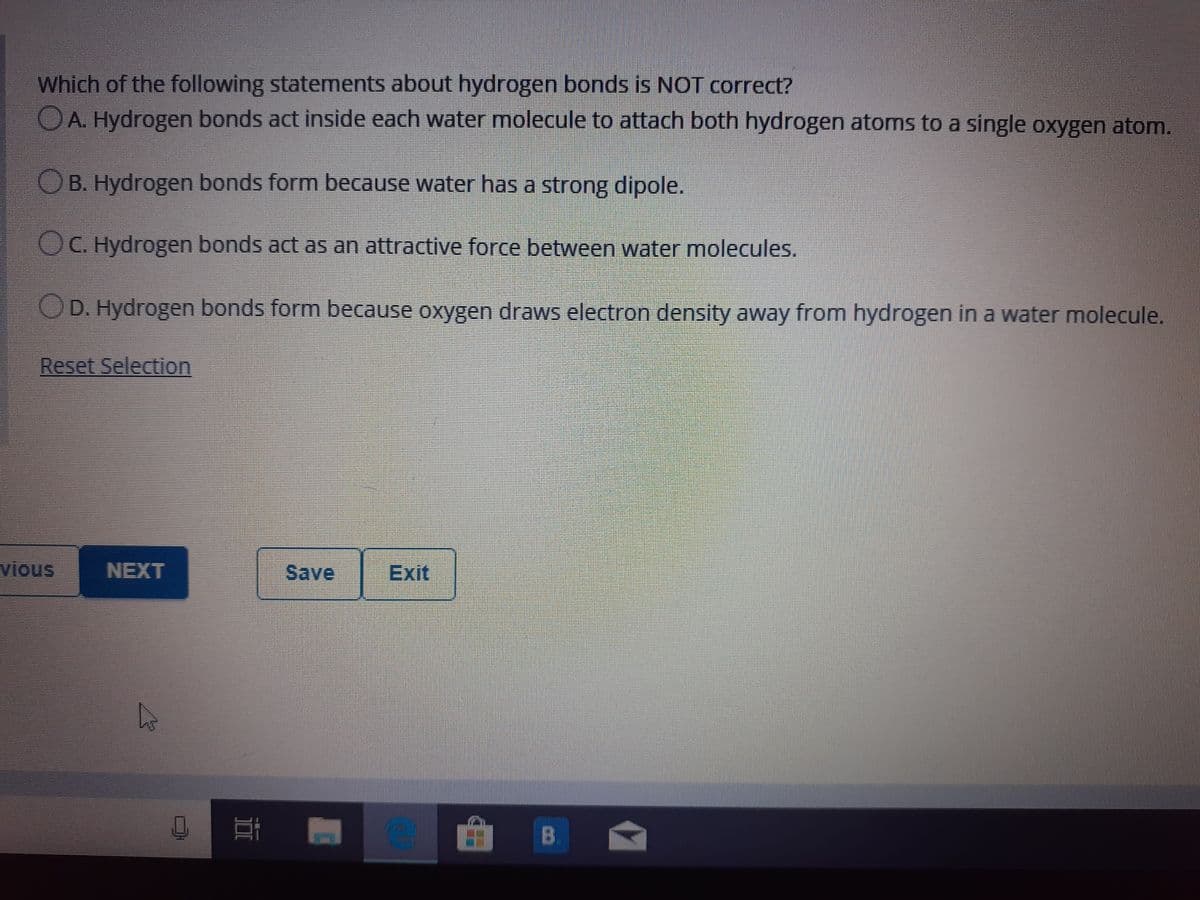
Transcribed Image Text:Which of the following statements about hydrogen bonds is NOT correct?
OA. Hydrogen bonds act inside each water molecule to attach both hydrogen atoms to a single oxygen atom.
OB. Hydrogen bonds form because water has a strong dipole.
OC. Hydrogen bonds act as an attractive force between water molecules.
OD. Hydrogen bonds form because oxygen draws electron density away from hydrogen in a water molecule.
Reset Selection
vious
NEXT
Save
Exit
B
DI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
