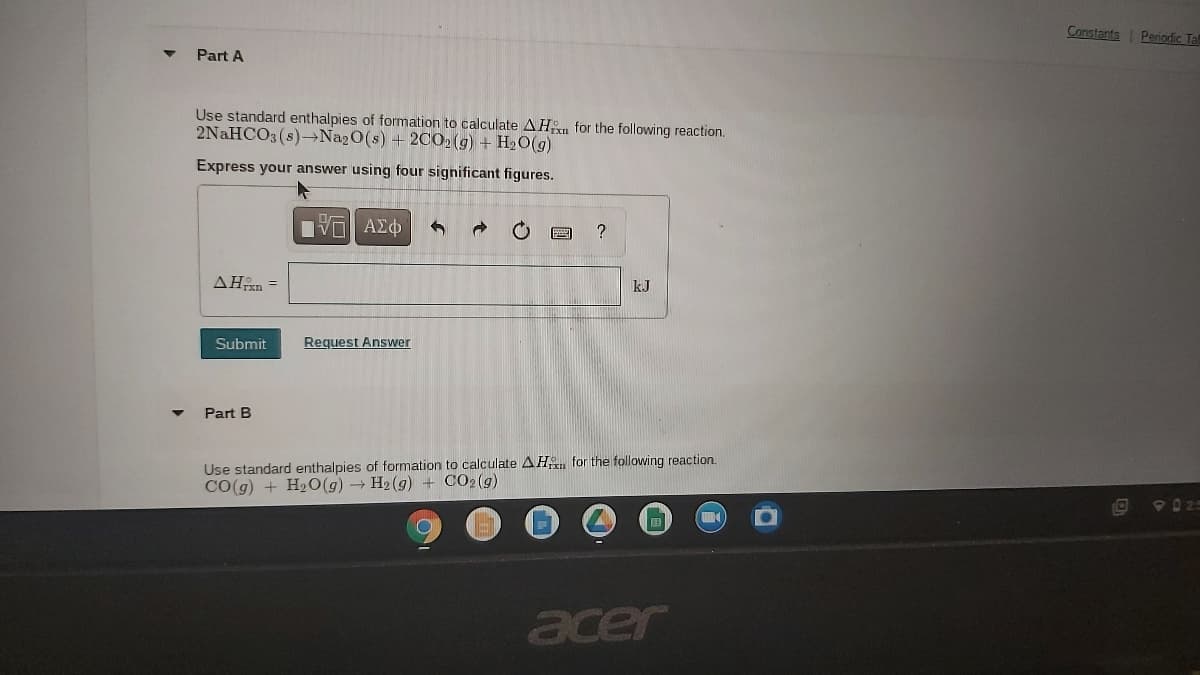
Com Part A Use standard enthalpies of formation to calculate AHn for the following reaction. 2NAHCO3 (s)→Na2O(s) + 2C02(9) + H2O(g) Express your answer using four significant figures. να ΑΣφ ? AHn = kJ Submit Request Answer Part B Use standard enthalpies of formation to calculate AH for the following reaction. CO(g) + H2O(g)H2(g) + CO2(g)
Com Part A Use standard enthalpies of formation to calculate AHn for the following reaction. 2NAHCO3 (s)→Na2O(s) + 2C02(9) + H2O(g) Express your answer using four significant figures. να ΑΣφ ? AHn = kJ Submit Request Answer Part B Use standard enthalpies of formation to calculate AH for the following reaction. CO(g) + H2O(g)H2(g) + CO2(g)
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter15: Energy And Chemical Change
Section: Chapter Questions
Problem 112A: sample of natural gas is analyzed and found to be88.4% methane (CH4) and 11.6% ethane (C2H6) bymass....
Related questions
Question
Transcribed Image Text:Constants | Periodic Tal
Part A
Use standard enthalpies of formation to calculate AHxn for the following reaction.
2NAHCO3 (s)-→Na20(s) + 2C02 (g) + H2O(g)
Express your answer using four significant figures.
?
AHn =
kJ
Submit
Request Answer
Part B
Use standard enthalpies of formation to calculate AH for the following reaction.
CO(g) + H2O(g) → H2 (g) + CO2(g)
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning