Combustion of hydrocarbons such as ethane (CH) produces carbon dioxide, a greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the s combustion of gaseous ethane into gaseous carbon dioxide and gaseous water. 0 2. Suppose 0.460 kg of ethane are burned in air at a pressure of exactly 1 atm and a temperature of 14.0 C. Calculate the volume of carbon dioxide gas that is produced Round your answer to 3 significant digits 0-0 00⁰ 80.2 X G
Combustion of hydrocarbons such as ethane (CH) produces carbon dioxide, a greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the s combustion of gaseous ethane into gaseous carbon dioxide and gaseous water. 0 2. Suppose 0.460 kg of ethane are burned in air at a pressure of exactly 1 atm and a temperature of 14.0 C. Calculate the volume of carbon dioxide gas that is produced Round your answer to 3 significant digits 0-0 00⁰ 80.2 X G
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter10: Quantity Relationships In Chemical Reactions
Section: Chapter Questions
Problem 62E
Related questions
Question
Give typed explanation
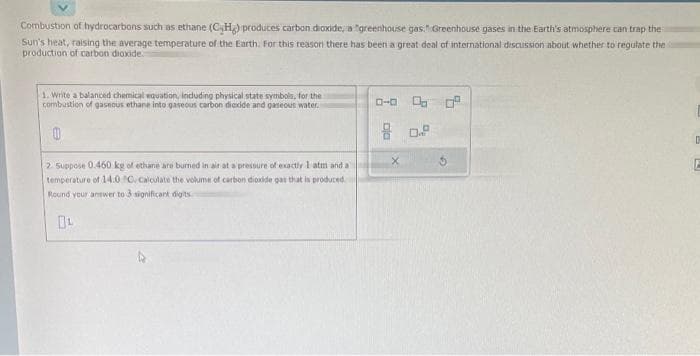
Transcribed Image Text:Combustion of hydrocarbons such as ethane (CH) produces carbon dioxide, a greenhouse gas Greenhouse gases in the Earth's atmosphere can trap the
Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the
production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the
combustion of gaseous ethane into gaseous carbon dioxide and gaseous water.
2. Suppose 0.460 kg of ethane are burned in air at a pressure of exactly I atm and a
temperature of 14.0 C. Calculate the volume of carbon dioxide gas that is produced
Round your answer to 3 significant digits
0-0 0.0 0²
80.2
X
D
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div