Compare the solubility of barium carbonate in each of the following aqueous solutions: Clear All 0.10 M Ba(NO,)2 More soluble than in pure water. 0.10 M Na,CO, Similar solubility as in pure water. 0.10 M NH,NO3 Less soluble than in pure water. 0.10 M NACH;CO0
Compare the solubility of barium carbonate in each of the following aqueous solutions: Clear All 0.10 M Ba(NO,)2 More soluble than in pure water. 0.10 M Na,CO, Similar solubility as in pure water. 0.10 M NH,NO3 Less soluble than in pure water. 0.10 M NACH;CO0
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.10QAP
Related questions
Question
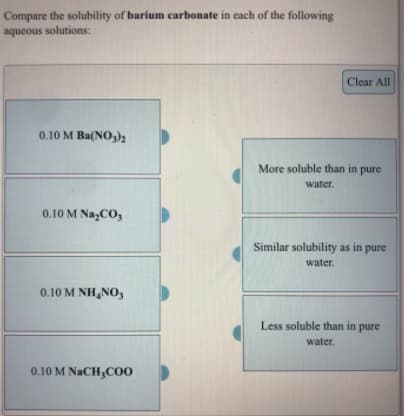
Transcribed Image Text:Compare the solubility of barium carbonate in each of the following
aqueous solutions:
Clear All
0.10 M Ba(NO,)2
More soluble than in pure
water.
0.10 M Na,CO,
Similar solubility as in pure
water.
0.10 M NH,NO3
Less soluble than in pure
water.
0.10 M NACH;CO0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning