Use the References to access important values if needed for this question. Each of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure silver bromide water. Similar solubility as in pure barium sulfite water. Less soluble than in pure magnesium fluoride water.
Use the References to access important values if needed for this question. Each of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure silver bromide water. Similar solubility as in pure barium sulfite water. Less soluble than in pure magnesium fluoride water.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 82CP
Related questions
Question
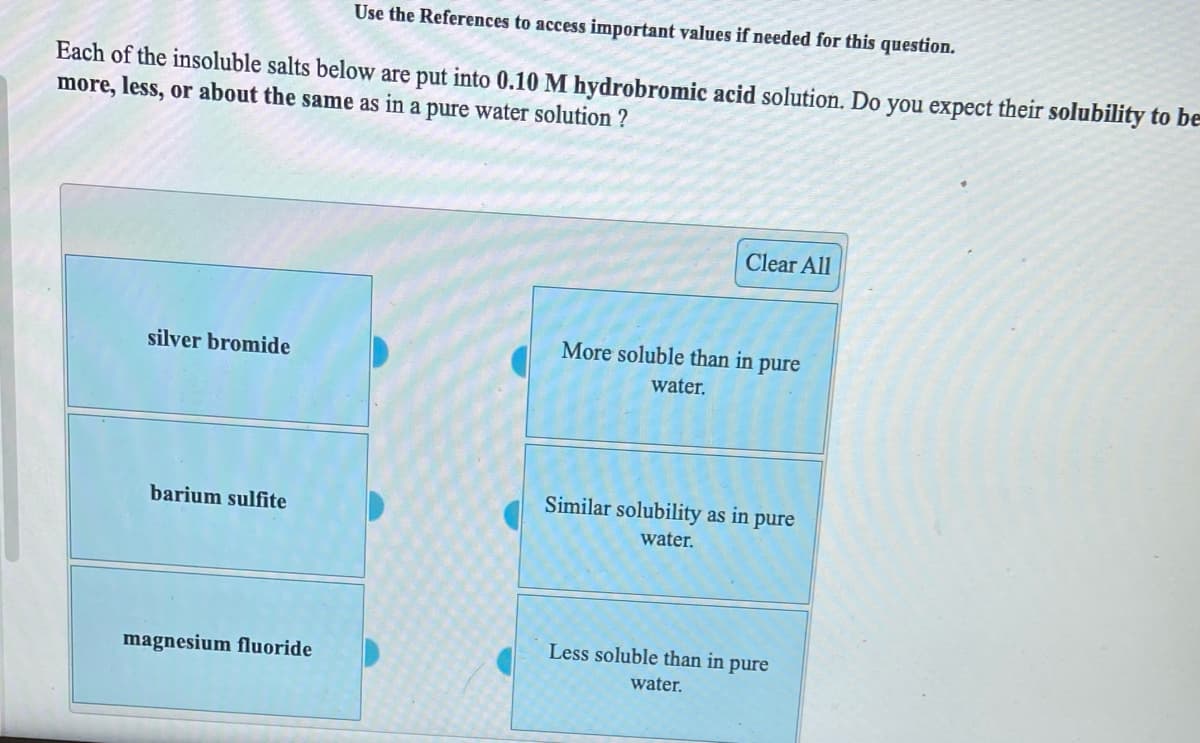
Transcribed Image Text:Use the References to access important values if needed for this question.
Each of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be
more, less, or about the same as in a pure water solution ?
Clear All
More soluble than in pure
silver bromide
water.
Similar solubility as in pure
barium sulfite
water.
Less soluble than in pure
magnesium fluoride
water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning




Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
