Compare the solubility of iron(II) carbonate in each of the following aqueous solutions: Clear All 0.10 M Fe(NO3)2 More soluble than in pure water. 0.10 MΝΗ)) CO, Similar solubility as in pure water. 0.10 M KCH3COO Less soluble than in pure water. 0.10 M NANO3
Compare the solubility of iron(II) carbonate in each of the following aqueous solutions: Clear All 0.10 M Fe(NO3)2 More soluble than in pure water. 0.10 MΝΗ)) CO, Similar solubility as in pure water. 0.10 M KCH3COO Less soluble than in pure water. 0.10 M NANO3
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.16QAP
Related questions
Question
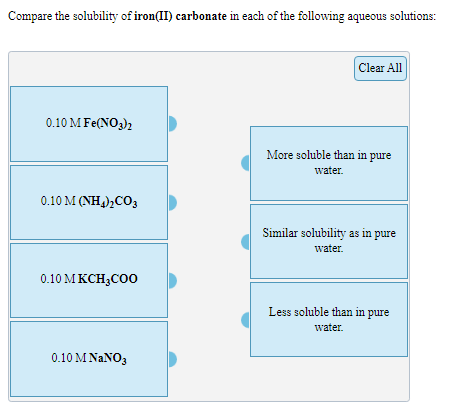
Transcribed Image Text:Compare the solubility of iron(II) carbonate in each of the following aqueous solutions:
Clear All
0.10 M Fe(NO3)2
More soluble than in pure
water.
0.10 M(Η)co,
Similar solubility as in pure
water.
0.10 M KCH;CO0
Less soluble than in pure
water.
0.10 M NaNΟ,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax