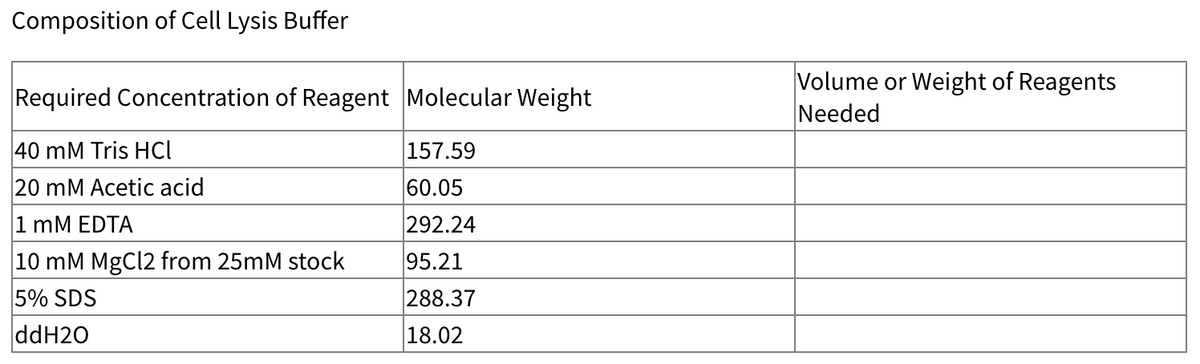
Composition of Cell Lysis Buffer Volume or Weight of Reagents Needed Required Concentration of Reagent Molecular Weight 40 mM Tris HCI 20 mM Acetic acid 157.59 60.05 1 mM EDTA 292.24 10 mM MgCl2 from 25mM stock 5% SDS ddH20 95.21 288.37 18.02
Q: Amoxicillin/clavulanate potassium (AUGMENTIN) powder for oral suspension is prepared prior to…
A: Amoxicillin-clavulanate potassium for oral suspension is generally used as an antibacterial and…
Q: A biochemist carefully measures the molarity of salt in 56. mL of photobacterium cell growth medium…
A: The initial volume of the cell growth medium is 56.0 ml, and the initial molarity of salt in the…
Q: Acetazolamide is a drug which inhibits carbonic anhydrase. Carbonic anhydrase participates in…
A: Carbonic anhydrase is inhibited by acetazolamide. A graph has been given in the question where…
Q: Phenylephrine and nafazolin solutions are used as decongestants to relieve nasal congestion. Your…
A: Here, 25% phenylephrine is available and now we have to calculate the amount needed to supply for…
Q: About defroxamine (all are correct except Select one: 1- its a tridentate chelating agent of…
A: A drug is any substance (except for food and water) that, when taken into the body, adjusts the…
Q: Graph of Absorbance vs Time(mins) 1.4 1.2 0.8 0.6 0.4 0.2 10 20 30 40 50 60 70 80 Time (mins) -Group…
A: growth rate : it is the rate at which the number of population of bacteria increases in a culture…
Q: Calculate the dimensionless spray flux for the normal case and the atypical batch, and explain why…
A: As given in the question, the value of spray flux for: Normal value - 0.52 Atypical value - 1.46…
Q: Menaquinones is produced by Flavobacterium meningosepticum via submerged fermentation with 60%…
A: Menaquinone are commonly known as vitamin K2. The structure of compound indicates that the compound…
Q: A purified protein is in a HEPES (N-(2-hydroxyethyl)piperazine-N-(2-ethanesulfonic acid)) buffer at…
A: In dialysis, a semipermeable membrane is used to isolate small molecules and proteins based on their…
Q: Prepare a standard tube that contains 0.2 mL of 2.5 mM lysine. To this tube, add 0.1 mL of the…
A: Final concentration can be calculated from the formula C1V1=C2V2 Where C1= initial concentration V1=…
Q: Ultrafiltration is often used for isolation of proteins from a fermentation broth. To make the…
A: Ultrafiltration is often used for isolation of proteins from a fermentation broth. To make the…
Q: A purified protein is in a Hepes (N-(2-hydroxy-ethyl)piperazine-N′-(2-ethanesulfonic acid)) buffer…
A: The kidneys extract waste materials out of the blood. Dialysis is a replacement of much of the…
Q: 5. Using this formula Cu = Au x Cstd. X CSstd Astd Where: C = concentration A = absorbance U =…
A: Given Values: Absorbance of the standard = 0.075 Concentration of the standard = 10.54 Absorbance…
Q: Vial No. A (ml) B (ml) C (ml) H20 1 2 5 Tris/HCl buffer pH7|1 1 1 Tumour cells (5% (w/v)) in buffer…
A: Biochemistry is the science of study of the internal biochemical processes of organisms. The…
Q: Recommend an appropriate dosage of digoxin for this patient given a trough digoxin concentration of…
A: Recommended appropriate level of digoxin is 0.5 to 2.0 ng/ml
Q: A 5% dextrose in 1/2 normal saline (D5 1/2 NS) solution is commonly administered to patients needing…
A: IV or Intravenous fluid when injected into patients has to be carefully regulated as it would…
Q: Blood must be maintained at a pH of 7.4. Since 7.4 is the best pH for blood, we describe it as a…
A: Answer: pH : It is measure of the concentration of hydrogen ions present in an aqueous solution OR,…
Q: Glycogen was isolated from a liver sample. Sixty milligrams of the crude glycogen were then…
A: Sugars with reducing property (having aldehyde or keto group) are called reducing sugars. Some…
Q: A glucose solution is supplied in a repeated fed batch culture. The following parameters have been…
A:
Q: You are asked to prepare HEPES buffered saline with the following constituents: НЕРES 10mM KCI 5mM…
A: The concentration of a solution is expressed in terms of the ratio of the amount of solute present…
Q: D-fructose (MW 180.16) is a nonelectrolyte and does not dissociate. Its dissociation factor is 1.…
A: Fructose is a simple ketogenic simple sugar found in many plants where it is often bonded to glucose…
Q: sample of adenosine triphosphate (AI nm) is placed cuvette with sufficient buffer to give a total…
A:
Q: a. The feed to the precipitation unit has 120 mg mAb/l and 28 mg impurity/l in 5 liters of product…
A: Given values in the question are as follows: mAb = 120 mgl Total volume of the solution = 5 l…
Q: Mike has determined that enzyme he is attempting to purify has an isoelectric point of 4.5 (pI =…
A: Enzymes are protein molecules that increase the rate of reaction by decreasing the activation…
Q: Enzyme kinteics of amylase using l2 in Kl was used to determine starch conc at t = 0 min and t = 1…
A: The standard curve allows estimating the concentration of unknown samples by comparing them to known…
Q: You have a sample at 50 ng/ul and you would like to load 400ng of this sample on a lane of an…
A: Agarose gel electrophoresis is a technique to study the features of genetic material and proteins.…
Q: Using native chromatographic conditions which preserve enzymatic activity, what would be the…
A: Gel filtration chromatography is also known as size exclusion chromatography. Gel filtration…
Q: Prescribed: Mesna 2,500 mg in 125 mL Normal Saline by IV to infuse at 250 mL/hr. On hand: vial…
A: Calculations play an important role in determining the amount of solution needed to take the right…
Q: Assume that you are starting with a sample containing 6 billion active cells. Create a dilution plan…
A: Active cells 6 billion Aim is to achieve 30-300 colonies per plate Sample weight 500 mg or 0.5 gm
Q: Calculate the of the acetic acid in for each – show all unit conversions (hint: start with mL of and…
A: The balanced chemical equation of vinegar with NaOH is as given below: CH3COOH + NaOH →CH3COONa +…
Q: a. The feed to the precipitation unit has 120 mg mAb/l and 28 mg impurity/l in 5 liters of product…
A: Given values in the question are as follows: mAb = 120 mgl Total volume of the solution = 5 l…
Q: Tirofiban (C22H36N2O5S MWt = 440.6) is present as tirofiban HCI monohydrate MWt=495.1) at 0.281…
A: •Given values •concentration of the solution= 0.281 mg/ml •gram of tirofiban in 50 ml adding…
Q: Composition of Cell Lysis Buffer
A: We will do calculations to prepare 1 L (1000 mL) of lysis buffer.
Q: you add &ml of baderial Suspensfon ath Concentration water 2x1d cellolmi to13 ml what is the new…
A: A serial dilution of a bacterial culture is the stepwise dilution of the culture in which the…
Q: Titanium dioxide, which is the most common white pigment in paint, can be produced from the titanium…
A: The ponchon- savarit method diagram for binary distillation allows by one to account for enthalpy…
Q: The glucose content of samples can be determined using Nelson't test. The equation of the line for…
A: Given : Standard curve of concentration vs absorbance equation, y = 3.0x + 0.2 Absorbance= 1.35
Q: ab Test omprehensive Metabolic anel Description Normal Values Clinical Significance if Above/below…
A: There are various metabolites present in the blood which have their normal values. lower and higher…
Q: Cyanide ion (CN-) causes toxicity to humans, explain the reason of its toxicity to us. Suggest a…
A: Cyanide is a compound that belongs to the cyano group. In this chemical compound, carbon is bonded…
Q: Paclitaxel is used to treat ovarian cancer by infusion at a concentration of 0.6 mg/mL over 24…
A: Given, Concentration of paclitaxel = 150mg/25mL. This means that 150mg of drug is present in 25 mL.…
Q: Calculate the amount of casein obteined from %20 milk solution with %2 HCl isoelectric point…
A: Protein precipitation is widely used in downstream processing of biological products in order to…
Q: Phosphate buffered saline (PBS) is a very common reagent in cell biology labs that do cell culture…
A: Phosphate-buffered saline (PBS) is a buffer of pH=7.4, that is commonly used in biological studies.…
Q: Calculate the concentration of p-aminophenol control to match the 0.075 % w/w limit in 200 mg/10 ml…
A: Given : 0.075% w/w limit in 200 mg/10 mL Asked : Concentration calculation.
Q: All of the following have been used to treat severe cases of COVID-19, EXCEPT Dexamethasone…
A: Given: All of the following have been used to treat severe cases of COVID-19.
Q: A biochemist carefully measures the molarity of salt in 56. mL of photobacterium cell growth medium…
A: GIVEN: The initial volume of the photobacterium cell growth medium (V1)= 56 mL Concentration of the…
Q: 5. Using this formula Cu = Au x Cstd Astd Where: C = concentration A = absorbance U = unknown/sample…
A: Blood glucose concentration is frequently tested from capillary blood taken from a finger prick.…
Q: Paclitaxel is used to treat ovarian cancer by infusion at a concentration of 0.6 mg/mL over 24…
A: Dose to be given = 135mg/m2 First calculate the Body Surface Area of the patient: Using mosteller…
Q: Convert the experimental glucose concentration from mM (millimolar) to g/100 mL. 1/100 Dilution…
A: Ans - Given : Molarity = 2.340 mM ~ 2.340 /1000 M Volume of solution = 1ltr ~ 1000 ml…
In this experiment, you are to prepare 250mL of Cell Lysis Buffer using the reagents given in Table. Fill out the table. Show your computations at the bottom of the table.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Composition of Cell Lysis Buffer Required Concentration of Reagent Molecular Weight Volume or Weight of Reagents Needed 40 mM Tris HCl 157.59 20 mM Acetic acid 60.05 1 mM EDTA 292.24 10 mM MgCl2 from 25mM stock 95.21 5% SDS 288.37 ddH2O 18.02The diffusivity of amino acids in polyacrylamide gel is approximately 1x10^-9 cm2./s calculate the initial flux of amino acids, give an instantaneous gradient of (20g/cm 3 )/8cm Why is polyacrylamide gel is used in electrophoresis?Infuse heparin at 1,200 units per hour from a solution containing 40,000 units of heparin in 500 mL D5W. How many mL/hr will deliver the ordered dosage?
- The isoelectric point of eIF4a is 5.02. Students are given a sample of cell lysate containing eIF4A at pH 7.4. They are also provided with two buffers of pH 7.4 to use for ion exchange chromatography. One buffer has a very high salt concentration (1 M), the other has a low salt concentration (0.1 M). Mariela decides to use an anion exchange column, while Ashok chooses a cation exchange column. Who made the better decision? Provide a detailed explanation of why one student will end up with a purer sample of eIF4A. Which buffer will you use while washing away impurities (high or low salt), and which would you use to remove eIF4A from the column? Explain your choice.after a 5 g onion sample is divided into two equal parts, heat treatment to one of these partsit is being implemented. Then both heat-treated and non-treated parts are 10 times their weightit is ground in an environment containing as much buffer and the determination of pyruvate in the supernatants obtainedit's being done.a) As a result of measurements taken at 520 nm; absorbance value for the heat-treated sample;it was found that the absorbance value in the sample that was not heat treated was 0.123 and the absorbance value in the sample that was not heat treated was 0.520.The equation of the calibration accuracy for pyruvate is that y = 0.1367x – 0.001 (nmol/mL)according to the onion sample; a) independent of alinase activity; b) dependent on alinase activityand c) calculate the total pyruvate amounts in terms of nmol/g of onion.b) How is there a relationship between the activity of the enzyme allinase in onions and the amount of pyruvate?Dec,please explain.Show the calculations required to make up 250mlof a stock solution of this chemical that will then be at the working concentration when 1 volume of this solution is added to 10 volumes of cell culture medium.
- what is the concetration of a lysozyme solution with an absorbance of 0.720 measured at 280 nm( e= 36000 -1M -1cm,l=1cm)A glucose solution is supplied in a repeated fed batch culture. The following parameters have been measured at t=2.5 hours; the system was under a quasi-steady state at this time. V = 1.0L, F = 0.22L/hr, S_0 = 98g/L, mu_m = 0.31/hr, K_s = 0.14 g-glucose/L, YM_X/S = 0.48 g-cells/g-glucose, Xt_0 = 33g (1) Calculate the value of V0 and S. (2) Calculate the value of Xt for t = 2.5hrs.Calculation at the initial point of a complexation titration: What is the pSr value of a 50.00 mL solution of 0.01000 M Sr2+ (formation constant, Kf = 5.348 x 108) after the addition of 0.00 mL of 0.02000 M EDTA which was buffered to pH 11.00? Round your final answer to two decimal places to the right of the decimal point.
- Dextrose 5% in water solution is an example of hypertonic solution. True False?The octapeptide gly-cys-met-asn-lys-ala-tyr-gly was hydrolyzed consecutively by CNBr and then trypsin (3 fragments total). The mixture of fragments was buffered at a pH 8.5 and then chromatographed on an anion-exchanger (positive resin) with the same buffer. Draw sequence (abbreviated form) of all fragments at that pH (show charges) and predict elution order.The protein calcineurin binds to the protein calmodulin with an association rate of 8.9 × 103 M−1 s −1 and an overall dissociation constant, Kd, of 10 nM. Calculate the dissociation rate, kd , including appropriate units.

