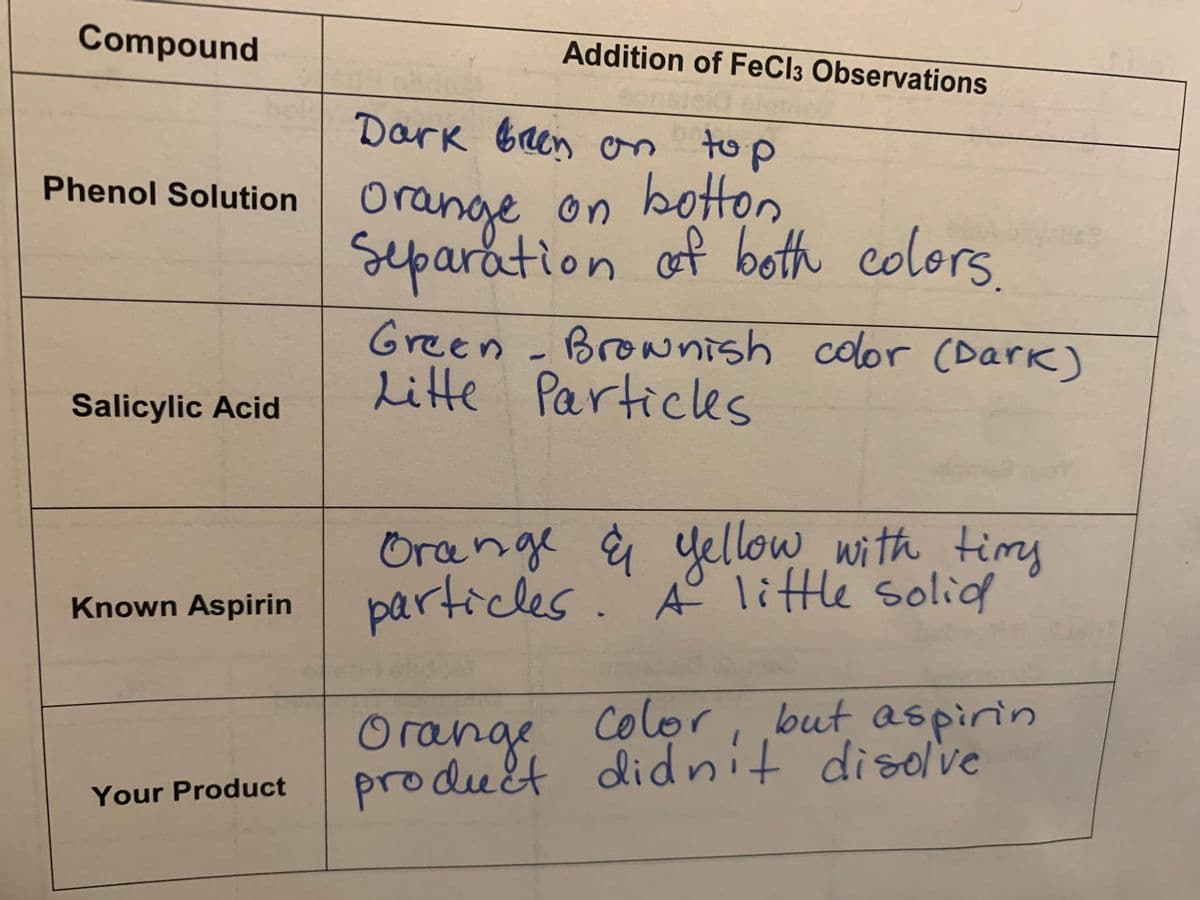
Compound Addition of FeCl3 Observations Dark ben on top botton Separation of both colors Phenol Solution Orange on Green - Brownish color (Dark) Litte Particles Salicylic Acid Orange & yellow with timy particles. A° little solid Known Aspirin Color, but, aspirin produét didnit disolve Orange Your Product
Compound Addition of FeCl3 Observations Dark ben on top botton Separation of both colors Phenol Solution Orange on Green - Brownish color (Dark) Litte Particles Salicylic Acid Orange & yellow with timy particles. A° little solid Known Aspirin Color, but, aspirin produét didnit disolve Orange Your Product
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.13QAP
Related questions
Question
Can you help me to support this?
Based on your observations of your TLC plate and FeCl3 tests, how pure is your aspirin?
Transcribed Image Text:particles
Compound
Addition of FeCl3 Observations
Dark bren on to p
on botton
Separation of both colors
Phenol Solution Orange on botton
Green - Brownish color (Dark)
Litte Particles
Salicylic Acid
Orange ģ yellow with timy
particles.' A° little solid
Known Aspirin
color, but aspirin
product didnit disolve
Orange
Your Product
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole