OBJECTIVES Prepare benzopinacol from benzophenone and isopropyl alcohol in the presence of light. I. Balanced equation for main reaction. II. Equation for secondary reaction (if any). IV. Performance calculations Limiting factor (name and moles) Theoretical yield moles_ Experimental yield in grams 10.044g - 9.753 g = 0.291 g -g
OBJECTIVES Prepare benzopinacol from benzophenone and isopropyl alcohol in the presence of light. I. Balanced equation for main reaction. II. Equation for secondary reaction (if any). IV. Performance calculations Limiting factor (name and moles) Theoretical yield moles_ Experimental yield in grams 10.044g - 9.753 g = 0.291 g -g
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 4E
Related questions
Question
1
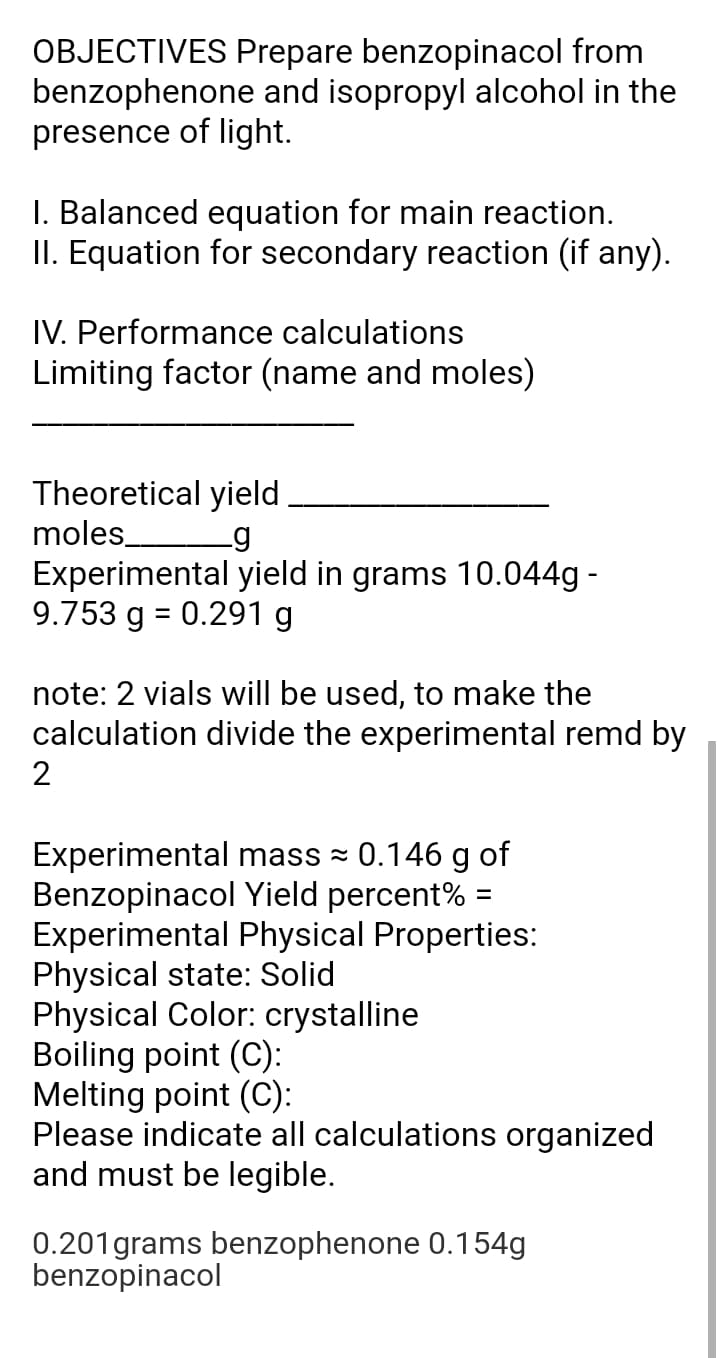
Transcribed Image Text:OBJECTIVES Prepare benzopinacol from
benzophenone and isopropyl alcohol in the
presence of light.
I. Balanced equation for main reaction.
II. Equation for secondary reaction (if any).
IV. Performance calculations
Limiting factor (name and moles)
Theoretical yield
moles
Experimental yield in grams 10.044g -
9.753 g = 0.291 g
note: 2 vials will be used, to make the
calculation divide the experimental remd by
2
Experimental mass = 0.146 g of
Benzopinacol Yield percent% =
Experimental Physical Properties:
Physical state: Solid
Physical Color: crystalline
Boiling point (C):
Melting point (C):
Please indicate all calculations organized
and must be legible.
0.201grams benzophenone 0.154g
benzopinacol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning