Conductive cell has resistance 22N when fill by 0.1m CH3COONA, 7.3N when fill by (0.1M CH3COONA+0.2M HCI) and 1.62 when using 0.1M NaCl solution and ^NaCl =126 · give: a. Cell constant b. Conductivity and molar conductivity for 0.1M CH3COONA c. Conductivity for solution (CH3COONA +HCl)
Conductive cell has resistance 22N when fill by 0.1m CH3COONA, 7.3N when fill by (0.1M CH3COONA+0.2M HCI) and 1.62 when using 0.1M NaCl solution and ^NaCl =126 · give: a. Cell constant b. Conductivity and molar conductivity for 0.1M CH3COONA c. Conductivity for solution (CH3COONA +HCl)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.23QAP: The following cell was found to have a potential of —0.492 V: Ag|AgCl(sat’d)||HA(0.200 M),NaA(0.300...
Related questions
Question
i need the answer quickly
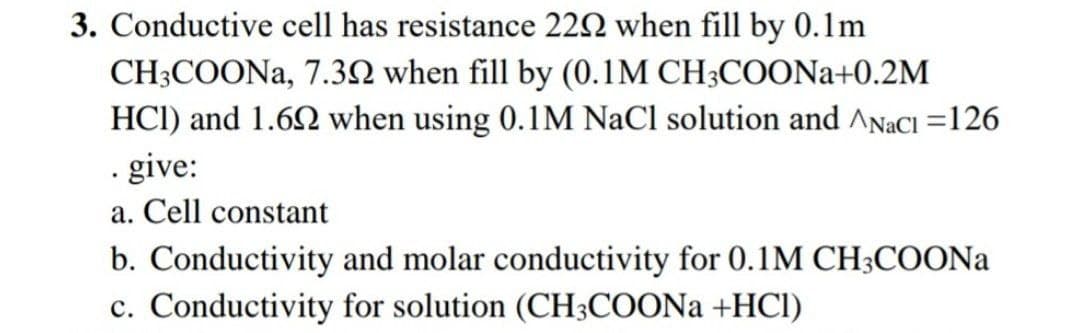
Transcribed Image Text:3. Conductive cell has resistance 222 when fill by 0.1m
CH3COONA, 7.32 when fill by (0.1M CH;COONA+0.2M
HCI) and 1.62 when using 0.1M NaCl solution and ANACI =126
· give:
a. Cell constant
b. Conductivity and molar conductivity for 0.1M CH;COONA
c. Conductivity for solution (CH3COONA +HCl)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

