Consider a protein in which a negatively charged glutamic acid side chain (pKa = 4.2) makes a salt bridge (ion-ion interaction) with a positively charged histidine side chain (pK₂ = 6.5). ▼ Part A Do you predict that this salt bridge will become stronger, become weaker, or be unaffected as pH increases from pH = 7.2 to pH = 7.8? The salt bridge will become stronger. The salt bridge will become weaker. The salt bridge will be unaffected. Submit Previous Answers ✓ Correct At pH = 7.2 the glutamic acid (Glu) side chain will carry a charge of ~ -1 (at 3 pH units above the pK₂ for Glu, the side chain will be almost fully ionized); whereas the histidine (His) side chain will carry a charge of < +0.5 (at pH=pK₂ the charge on His would be +0.5; since pH = 7.2 is above its pKa, it will carry less (+) charge as it becomes more deprotonated). As the pH increase to 7.8, the charge on Glu will remain ~ -1 and the charge on His will decrease; thus, this salt bridge is predicted to become weaker as pH is raised from 7.2 to 7.8. Part B Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.2. (Hint: Consider lessons from Coulomb's law, and the Henderson- Hasselbalch equation.) Express your answers using two significant figures separated by a comma. 1ΨΕΙ ΑΣΦ ?
Consider a protein in which a negatively charged glutamic acid side chain (pKa = 4.2) makes a salt bridge (ion-ion interaction) with a positively charged histidine side chain (pK₂ = 6.5). ▼ Part A Do you predict that this salt bridge will become stronger, become weaker, or be unaffected as pH increases from pH = 7.2 to pH = 7.8? The salt bridge will become stronger. The salt bridge will become weaker. The salt bridge will be unaffected. Submit Previous Answers ✓ Correct At pH = 7.2 the glutamic acid (Glu) side chain will carry a charge of ~ -1 (at 3 pH units above the pK₂ for Glu, the side chain will be almost fully ionized); whereas the histidine (His) side chain will carry a charge of < +0.5 (at pH=pK₂ the charge on His would be +0.5; since pH = 7.2 is above its pKa, it will carry less (+) charge as it becomes more deprotonated). As the pH increase to 7.8, the charge on Glu will remain ~ -1 and the charge on His will decrease; thus, this salt bridge is predicted to become weaker as pH is raised from 7.2 to 7.8. Part B Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.2. (Hint: Consider lessons from Coulomb's law, and the Henderson- Hasselbalch equation.) Express your answers using two significant figures separated by a comma. 1ΨΕΙ ΑΣΦ ?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
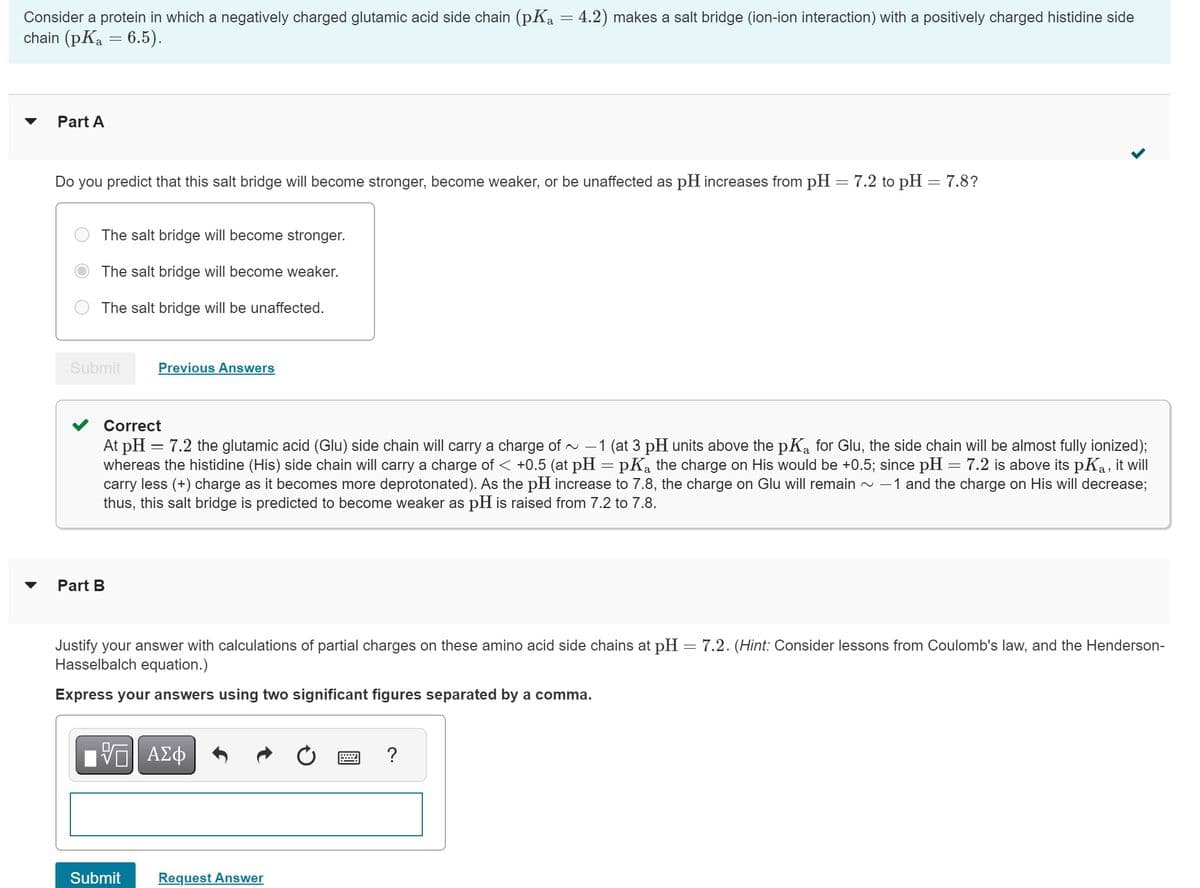
Transcribed Image Text:Consider a protein in which a negatively charged glutamic acid side chain (pKa = 4.2) makes a salt bridge (ion-ion interaction) with a positively charged histidine side
chain (pKa = 6.5).
Part A
Do you predict that this salt bridge will become stronger, become weaker, or be unaffected as pH increases from pH = 7.2 to pH = 7.8?
The salt bridge will become stronger.
The salt bridge will become weaker.
The salt bridge will be unaffected.
Submit
Part B
Previous Answers
Correct
At pH = 7.2 the glutamic acid (Glu) side chain will carry a charge of ~ -1 (at 3 pH units above the pKa for Glu, the side chain will be almost fully ionized);
whereas the histidine (His) side chain will carry a charge of < +0.5 (at pH = pK₂ the charge on His would be +0.5; since pH = 7.2 is above its pKa, it will
carry less (+) charge as it becomes more deprotonated). As the pH increase to 7.8, the charge on Glu will remain ~ -1 and the charge on His will decrease;
thus, this salt bridge is predicted to become weaker as pH is raised from 7.2 to 7.8.
Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.2. (Hint: Consider lessons from Coulomb's law, and the Henderson-
Hasselbalch equation.)
Express your answers using two significant figures separated by a comma.
FVTI ΑΣΦ
Submit
<
Request Answer
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON