Consider The Following Reaction M Inbox (208) - eleganceextreme1 X + 05657571&snapshotld=13245488id-563649000&takeld-4bfba59b09f0e5b& X X QSearch this course X References Use the References to access important values if d for this question. *** The equilibrium constant, K,, for the following reaction is 8.46x10 at 298 K: 1 00 NH3(g) + H2Se(g) NH4HSe(s) A-Z Calculate the partial pressure of each gas and the total pressure at equilibrium when 0.469 moles of NHHSe(s) is introduced into a 1.00 L vessel at 298 K. Assume that the volume occupied by the solid is negligible. PNH3 atm atm PH2SE atm Ptotal 6. Equilibrium Calculations with Partial Pressur... : This is group attempt 1 of 5 Next Autosaved at 1:06 PM Back 1:06 PM xa 11 9/24/2019 1 hp delete rt co
Consider The Following Reaction M Inbox (208) - eleganceextreme1 X + 05657571&snapshotld=13245488id-563649000&takeld-4bfba59b09f0e5b& X X QSearch this course X References Use the References to access important values if d for this question. *** The equilibrium constant, K,, for the following reaction is 8.46x10 at 298 K: 1 00 NH3(g) + H2Se(g) NH4HSe(s) A-Z Calculate the partial pressure of each gas and the total pressure at equilibrium when 0.469 moles of NHHSe(s) is introduced into a 1.00 L vessel at 298 K. Assume that the volume occupied by the solid is negligible. PNH3 atm atm PH2SE atm Ptotal 6. Equilibrium Calculations with Partial Pressur... : This is group attempt 1 of 5 Next Autosaved at 1:06 PM Back 1:06 PM xa 11 9/24/2019 1 hp delete rt co
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter13: An Introduction To Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 13.12QAP: The equilibrium constant for the reaction 2CrO42+2H+Cr2O72+H2O is 4.2 1014. The molar...
Related questions
Question
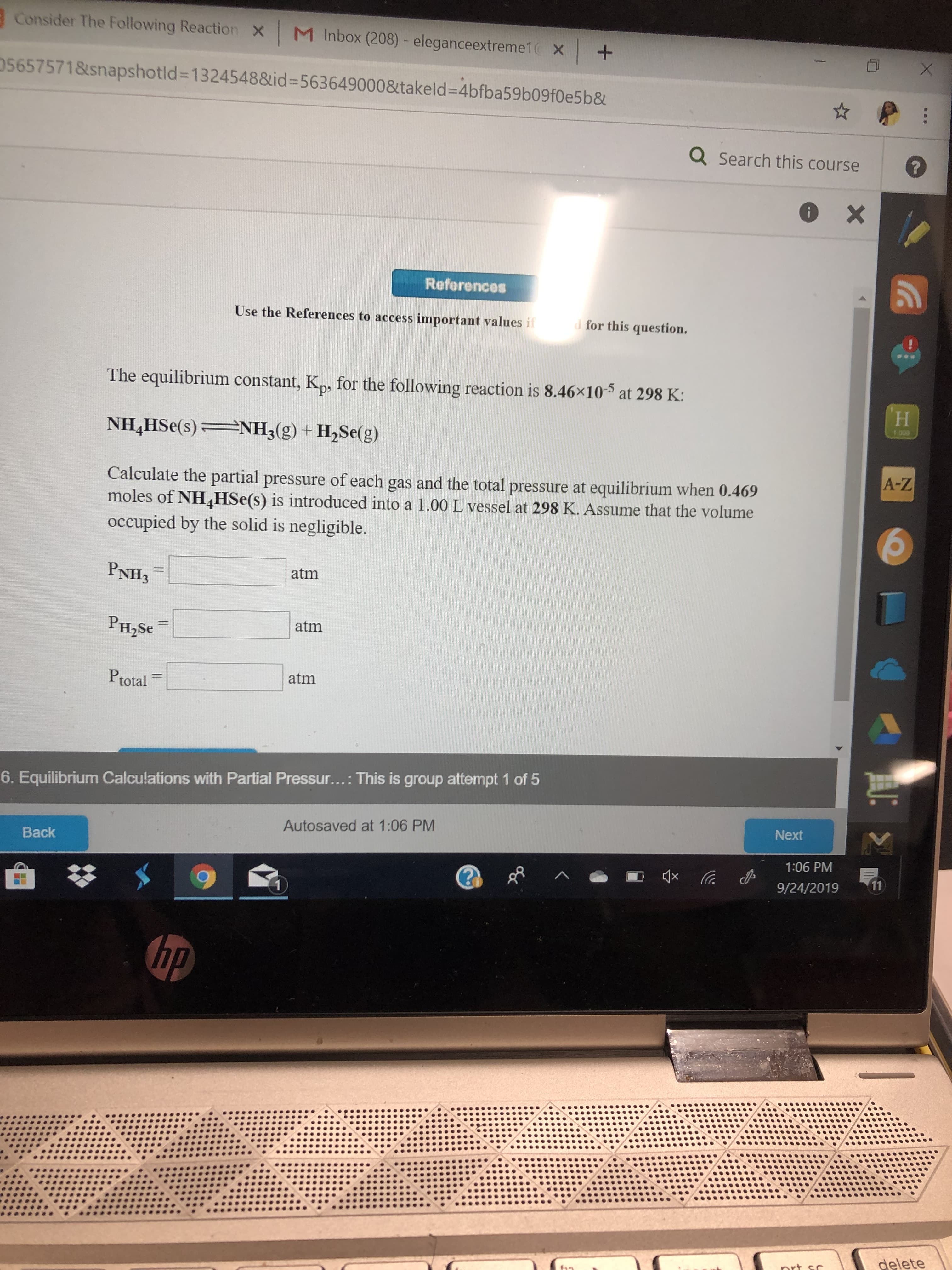
Transcribed Image Text:Consider The Following Reaction
M Inbox (208) - eleganceextreme1
X
+
05657571&snapshotld=13245488id-563649000&takeld-4bfba59b09f0e5b&
X
X
QSearch this course
X
References
Use the References to access important values if
d for this question.
***
The equilibrium constant, K,, for the following reaction is 8.46x10
at 298 K:
1 00
NH3(g) + H2Se(g)
NH4HSe(s)
A-Z
Calculate the partial pressure of each gas and the total pressure at equilibrium when 0.469
moles of NHHSe(s) is introduced into a 1.00 L vessel at 298 K. Assume that the volume
occupied by the solid is negligible.
PNH3
atm
atm
PH2SE
atm
Ptotal
6. Equilibrium Calculations with Partial Pressur... : This is group attempt 1 of 5
Next
Autosaved at 1:06 PM
Back
1:06 PM
xa
11
9/24/2019
1
hp
delete
rt co
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1
VIEWTrending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning