The triple bond in the N2 molecule is very strong, but at high enough temperatures even it breaks down. At 5000 K, when the total pressure exerted by a sample of nitrogen is 1.00 atm, N2(g) is 0.65% dissociated at equilibrium: N2(g) 2 2 N(g) At 6000 K with the same total pressure, the proportion of N,(g) dissociated at equilibrium rises to 11.6%. Use the van't Hoff equation to estimate the AH of this reaction.
The triple bond in the N2 molecule is very strong, but at high enough temperatures even it breaks down. At 5000 K, when the total pressure exerted by a sample of nitrogen is 1.00 atm, N2(g) is 0.65% dissociated at equilibrium: N2(g) 2 2 N(g) At 6000 K with the same total pressure, the proportion of N,(g) dissociated at equilibrium rises to 11.6%. Use the van't Hoff equation to estimate the AH of this reaction.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 34QAP: At 500C, k for the for the formation of ammonia from nitrogen and hydrogen gases is 1.5105....
Related questions
Question
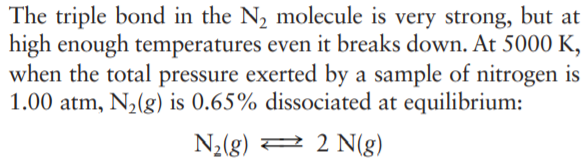
Transcribed Image Text:The triple bond in the N2 molecule is very strong, but at
high enough temperatures even it breaks down. At 5000 K,
when the total pressure exerted by a sample of nitrogen is
1.00 atm, N2(g) is 0.65% dissociated at equilibrium:
N2(g) 2 2 N(g)

Transcribed Image Text:At 6000 K with the same total pressure, the proportion
of N,(g) dissociated at equilibrium rises to 11.6%. Use
the van't Hoff equation to estimate the AH of this
reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning