Consider the molecular orbital diagram below when answering the following questions. Make sure to answer all 3 questions. a) What is the bond order for this species? b) Is this species paramagnetic or diamagnetic? c) Which neutral diatomic molecule does this MO diagram represent? 2p 2p 12p O2p 2s 2s
Consider the molecular orbital diagram below when answering the following questions. Make sure to answer all 3 questions. a) What is the bond order for this species? b) Is this species paramagnetic or diamagnetic? c) Which neutral diatomic molecule does this MO diagram represent? 2p 2p 12p O2p 2s 2s
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 17P
Related questions
Question
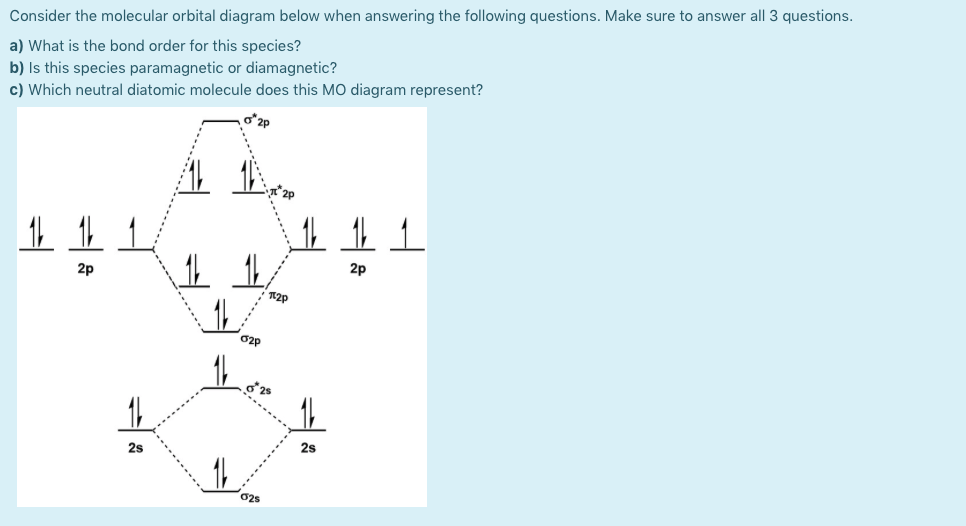
Transcribed Image Text:Consider the molecular orbital diagram below when answering the following questions. Make sure to answer all 3 questions.
a) What is the bond order for this species?
b) Is this species paramagnetic or diamagnetic?
c) Which neutral diatomic molecule does this MO diagram represent?
2p
2p
12p
O2p
2s
2s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 6 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
