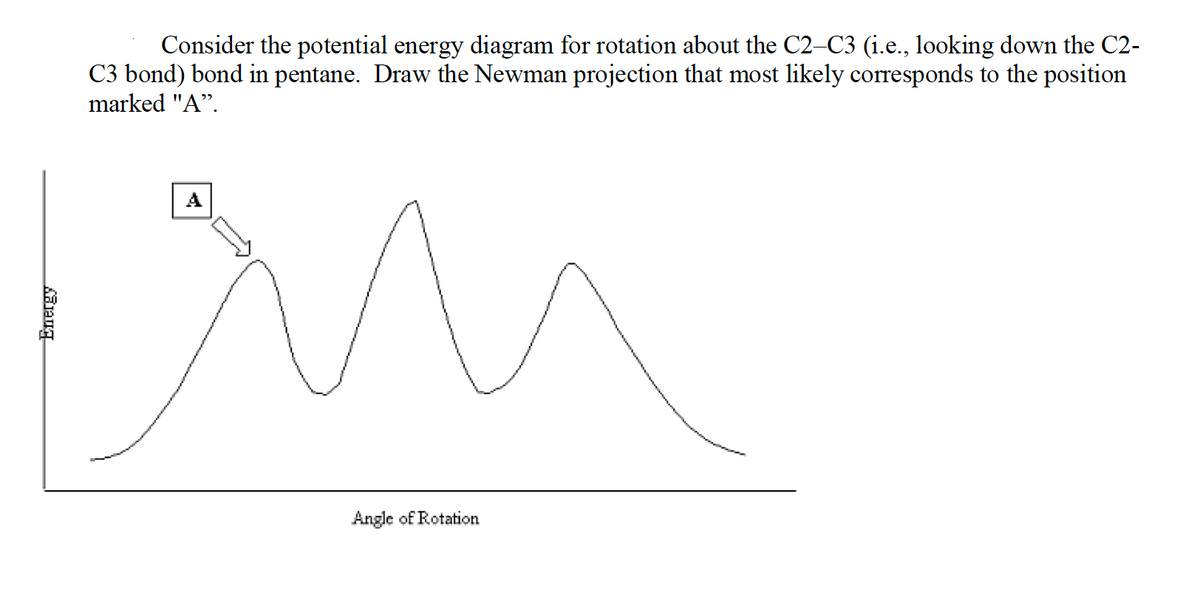
Consider the potential energy diagram for rotation about the C2-C3 (i.e., looking down the C2- C3 bond) bond in pentane. Draw the Newman projection that most likely corresponds to the position marked "A". A Angle of Rotation
The given problem deals with drawing Newman projection of a hydrocarbon. Newman projection is one of the most convenient methods of drawing a 3-D molecule on a paper using some specific set of rules which are :
i) Two tetrahedral atoms are taken to be as our reference.
ii) One of the two atoms is chosen as the front atom and is represented by drawing a dot.
iii) The other atom now becomes a rear atom and is represented by drawing a circle over the dot(front atom).
iv) The bonds attached to the front atoms are drawn as directly connecting to the dot.
v) The bonds connected to the rear atom are drawn connecting to the edges of the circle.
vi) The molecule is looked as if we are looking it directly from the front atom so that the rear atom hides behind it and we can get a clear stereochemical arrangements of all the atoms/bonds in the molecule.
Potential energy diagram shows the relative stability/ energy of a particular conformer against the dihedral angle. Now, dihedral angle is the angle between bonds on the front and back atoms. There are two major type of conformers with respect to the dihedral angles between bonds on front and rear atoms.
i) - Staggered Conformer - This is the most stable arrangement of the molecule and is generally observed to be at the bottom of the potential energy diagram or a minimum. Here, the bulky groups are as far as possible from each other and thus there is minimum steric strain and hence lower energy, so lies on bottom of Potential Energy (PE)curve.
ii) Eclipsed Conformer- This is the most unstable arrangement of the molecule and is generally observed to be a maximum in the PE curve. Here the bulky groups overlaps each other and thus there is high steric strain and thus have high energy and lies at maximum in PE.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images


