
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 17E
Build a model of methylcyclohexane, and use the model to complete the following Newmanprojections of methylcyclohexane in the chair conformation: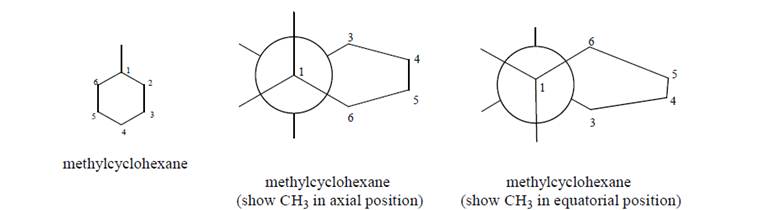 a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation.
a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation.
b. Label one Newman projection above anti and the other gauche to describe the relationshipbetween the methyl group and
c. In general, which is a lower PE conformation, anti or gauche?
d. Explain how your answer to b and c provide an explanation for why it is more favorable fora large group to be in an equatorial than an axial position.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Then draw the most stable and least stable Newman projection conformation from the C4-C5 bond in the molecule above
a) Sighting down the C3-C4 bond, draw the gauche (60 degrees) and anti (180 degrees) Newman projections of 2,4-dimethylhexane.
b) Circle the conformation that you drew that is lower energy.
Consider the substituted cyclohexane shown in the ball-and-stick model.a.Label the substituents on C1, C2, and C4 as axial or equatorial.
b. Are the substituents on C1 and C2 cis or trans to each other?
c.Are the substituents on C2 and C4 cis or trans to each other?
d.Draw the second possible conformation in the chair form, and classify it as more stable or less stable than the conformation shown in the three-dimensional model.
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 7 - Prob. 1CTQCh. 7 - Prob. 2CTQCh. 7 - Prob. 3CTQCh. 7 - Draw wedge and dash skeletal representations of...Ch. 7 - Label each ring in Figure 7.2 cis or trans.Ch. 7 - Prob. 6CTQCh. 7 - Prob. 7CTQCh. 7 - Prob. 8CTQCh. 7 - a model of cyclohexane in a chair conformation,...Ch. 7 - Prob. 10CTQ
Ch. 7 - Prob. 11CTQCh. 7 - Fill in the blanks: cis-1,3-Dimethylcyclohexane...Ch. 7 - Prob. 13CTQCh. 7 - Prob. 14CTQCh. 7 - Prob. 15CTQCh. 7 - Prob. 16CTQCh. 7 - Prob. 17CTQCh. 7 - Prob. 18CTQCh. 7 - Draw chair representations of...Ch. 7 - Which stereoisomer in the previous question is...Ch. 7 - Prob. 21CTQCh. 7 - Prob. 1ECh. 7 - Label each of the following as cis, trans or...Ch. 7 - Which pair has more in common with one another?Ch. 7 - Prob. 4ECh. 7 - Prob. 5ECh. 7 - Prob. 6ECh. 7 - Fill in the table by drawing a representation of a...Ch. 7 - Prob. 9ECh. 7 - True or False: If you perform a chair flip on...Ch. 7 - Prob. 11ECh. 7 - Prob. 12ECh. 7 - Prob. 13ECh. 7 - Prob. 14ECh. 7 - Prob. 15ECh. 7 - Draw trans-1-tert-butyl-3-methylcyclohexane in its...Ch. 7 - Build a model of methylcyclohexane, and use the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is a chair conformation of cyclohexane with the carbon atoms numbered 1 through 6. (a) Draw hydrogen atoms that are above the plane of the ring on carbons 1 and 2 and below the plane of the ring on carbon 4. (b) Which of these hydrogens are equatorial? Which are axial? (c) Draw the alternative chair conformation. Which hydrogens are equatorial? Which are axial? Which are above the plane of the ring? Which are below it?arrow_forwardConsider the molecule 1-bromo-2-methylbutane. C3 and C4 should be drawn as Et as in theexample. This group is called an ethyl group and can be considered a sphere about twice the sizeof a methyl group. Draw the following Newman projections sighting down the C1C2 bond... a. The lowest potential energy conformation. b. The highest potential energy staggered conformation.arrow_forwardOn the left is a stereorepresentation of glucose (we discuss the structure and chemistry of glucose in Chapter 25). (a) Convert the stereorepresentation on the left to a planar hexagon representation. (b) Convert the stereorepresentation on the left to a chair conformation. Which substituent groups in the chair conformation are equatorial? Which are axial?arrow_forward
- Consider the Newman projection below. a. Draw a full Lewis structure of this molecule with R1=Me,R2=Et , and R3=iPr . b. Given the sizes of these R groups (R3R2R1) , does the Newman projection above show thelowest potential energy conformation of this bond? If not, draw a Newman projectionshowing the lowest P.E. conformation (sighting down this same bond). c. To draw a Newman projection in the lowest P.E. conformation, the following rule of thumbusually applies: Place the largest group on the front carbon anti to the largest group on theback carbon. Is your answer to the previous question consistent with this rule of thumb?arrow_forwardFill in the blanks: cis-1,3-Dimethylcyclohexane has two different chair conformations: one withboth methyl groups in __________ positions and one with both methyl groups in ____________ positions.arrow_forwarda model of cyclohexane in a chair conformation, and explain why the names “axial” and“equatorial’ are appropriate.arrow_forward
- Use your answers from Problem 2.48 to complete the table showing correlations between cis,trans and axial,equatorial for disubstituted derivatives of cyclohexane. Draw the alternative chair conformations for the cis and trans isomers of 1,2-dimethylcyclohexane, 1,3-dimethylcyclohexane, 1,4-dimethylcyclohexane. (a) Indicate by a label whether each methyl group is axial or equatorial.arrow_forwardConsider the substituted cyclohexane shown in the ball-and-stick model.a. Label the substituents on C1, C2, and C4 as axial or equatorial.b. Are the substituents on C1 and C2 cis or trans to each other?c. Are the substituents on C2 and C4 cis or trans to each other?d. Draw the second possible conformation in the chair form, and classify it as more stable or less stable than the conformation shown in the three-dimensional model.arrow_forwardConstruction a qualitative potential energy for rotation about the C C Bond of 1,2-dibromoethane. Which conformation Would you expect to be more stabel? Label the anti and gauche conformations of 1,2 -dibrimoethanaarrow_forward
- For pentane draw Newman projections for the Syn-periplanar, conformation. the Anti- periplanar conformation and a Gauche conformation. Use C2 as the front carbon and C3 as the back carbon. Label each conformation, circle the highest energy conformation andunderline the lowest energy conformation.arrow_forwardDraw 2,4-dimethylhexane as a bond-line structure. Then, looking down the C3 – C4 bond, draw a Newman projection representation of the 1) lowest energy (most stable) and 2) highest energy (least stable) conformations.arrow_forwardAssign the absolute configuration of all molecules. Draw the most stable conformer in Newman projects with respect to bond C2-C3; for both a) and b). Draw Fisher projections for all molecules.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License