Consider the reaction: 2 HBr(g) H2(8) + Br2(8) a. Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. b. In the first 25.0 s of this reaction, the concentration of HBr dropped from 0.600 M to 0.512 M. Calculate the average rate of the reaction during this time interval. c. If the volume of the reaction vessel in part b was 1.50 L, what amount of Br2 (in moles) was formed during the first 15.0 s of the reaction?
Consider the reaction: 2 HBr(g) H2(8) + Br2(8) a. Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. b. In the first 25.0 s of this reaction, the concentration of HBr dropped from 0.600 M to 0.512 M. Calculate the average rate of the reaction during this time interval. c. If the volume of the reaction vessel in part b was 1.50 L, what amount of Br2 (in moles) was formed during the first 15.0 s of the reaction?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 100AE: Consider a hypothetical reaction between A and B: A + B products Use the following initial rate...
Related questions
Question
100%
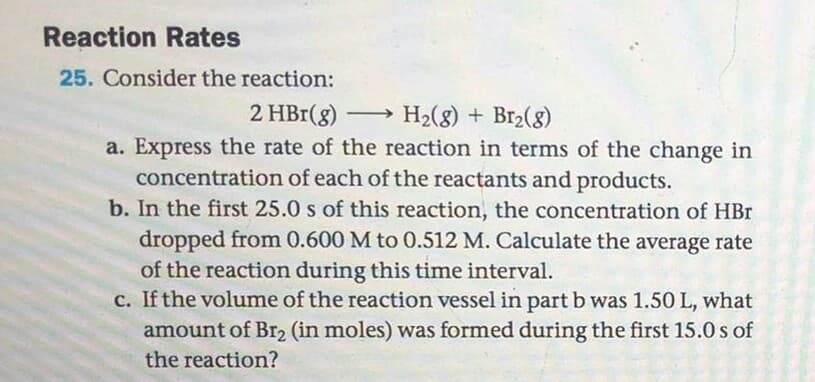
Transcribed Image Text:Consider the reaction:
2 HBr(g) H2(8) + Br2(8)
a. Express the rate of the reaction in terms of the change in
concentration of each of the reactants and products.
b. In the first 25.0 s of this reaction, the concentration of HBr
dropped from 0.600 M to 0.512 M. Calculate the average rate
of the reaction during this time interval.
c. If the volume of the reaction vessel in part b was 1.50 L, what
amount of Br2 (in moles) was formed during the first 15.0 s of
the reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning