Consider the reaction: 2NH3(g) + 202(g) → N₂O(g) + 3H₂O(!) Using standard thermodynamic data at 298 K, calculate the entropy change for the surroundings when 1.85 moles of NH3 (9) react at standard conditions. Substance AH (kJ/mol) NH₁(9) -46.1 02 (9) 0.0 N₂O(g) 82.1 H₂O(!) -285.8 J/K AS° surroundings
Consider the reaction: 2NH3(g) + 202(g) → N₂O(g) + 3H₂O(!) Using standard thermodynamic data at 298 K, calculate the entropy change for the surroundings when 1.85 moles of NH3 (9) react at standard conditions. Substance AH (kJ/mol) NH₁(9) -46.1 02 (9) 0.0 N₂O(g) 82.1 H₂O(!) -285.8 J/K AS° surroundings
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter13: Spontaneous Processes And Thermodynamic Equilibrium
Section: Chapter Questions
Problem 46AP
Related questions
Question
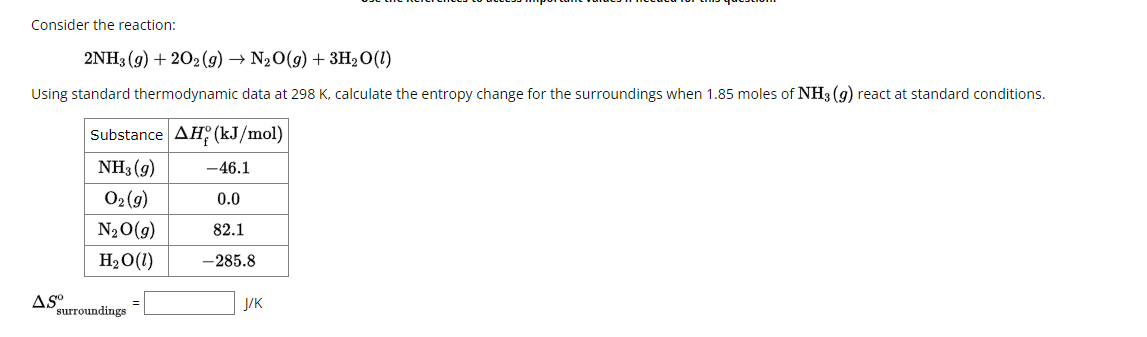
Transcribed Image Text:Consider the reaction:
2NH3(g) + 202(g) → N₂O(g) + 3H₂O(!)
Using standard thermodynamic data at 298 K, calculate the entropy change for the surroundings when 1.85 moles of NH3 (9) react at standard conditions.
Substance AH (kJ/mol)
NH₁(9)
-46.1
02 (9)
0.0
N₂O(g)
82.1
H₂O(!)
-285.8
J/K
AS°
surroundings
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning