Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH=2. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than one molecule is unstable, you can pick any of them to redraw.) OH OH HO Ostable Ounstable O stable Ounstable HO Click and drag to start drawing a structure. stable Ounstable O stable Ounstable ☐ X 口: G EX olo
Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH=2. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than one molecule is unstable, you can pick any of them to redraw.) OH OH HO Ostable Ounstable O stable Ounstable HO Click and drag to start drawing a structure. stable Ounstable O stable Ounstable ☐ X 口: G EX olo
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 29E
Related questions
Question
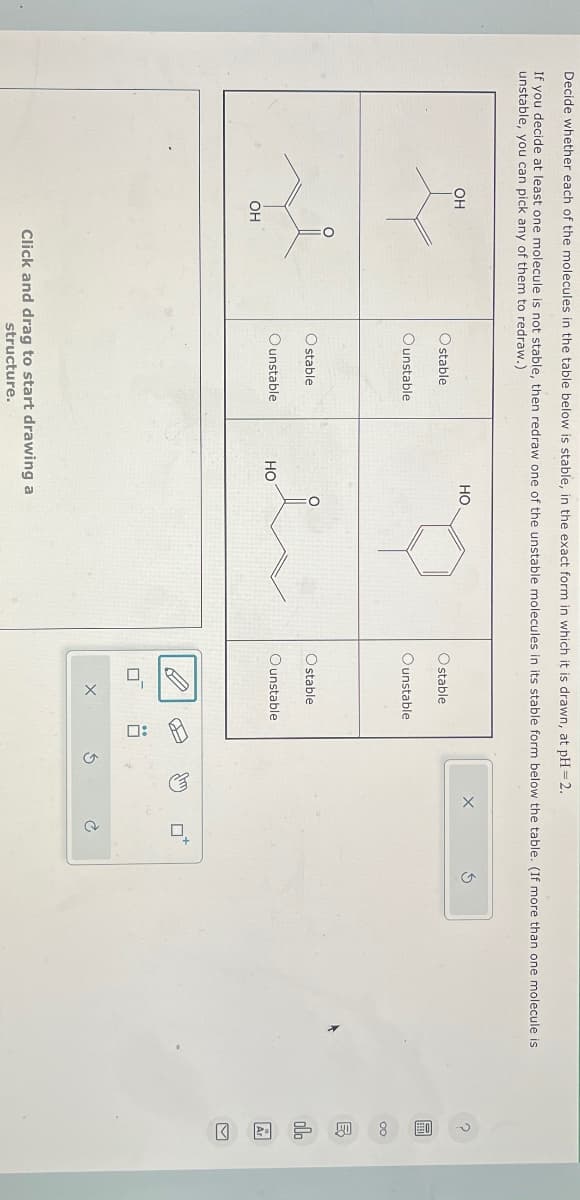
Transcribed Image Text:Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH=2.
If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than one molecule is
unstable, you can pick any of them to redraw.)
OH
OH
HO
Ostable
Ounstable
O stable
Ounstable
HO
Click and drag to start drawing a
structure.
stable
Ounstable
O stable
Ounstable
☐
X
口:
G
EX
olo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning