Consider the reaction. 2 Pb(s) + O,(g) → 2 PbO(s) An excess of oxygen reacts with 451.4 g of lead, forming 343.8 g of lead(II) oxide. Calculate the percent yield of the reaction. percent yield:
Consider the reaction. 2 Pb(s) + O,(g) → 2 PbO(s) An excess of oxygen reacts with 451.4 g of lead, forming 343.8 g of lead(II) oxide. Calculate the percent yield of the reaction. percent yield:
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 14CR
Related questions
Question
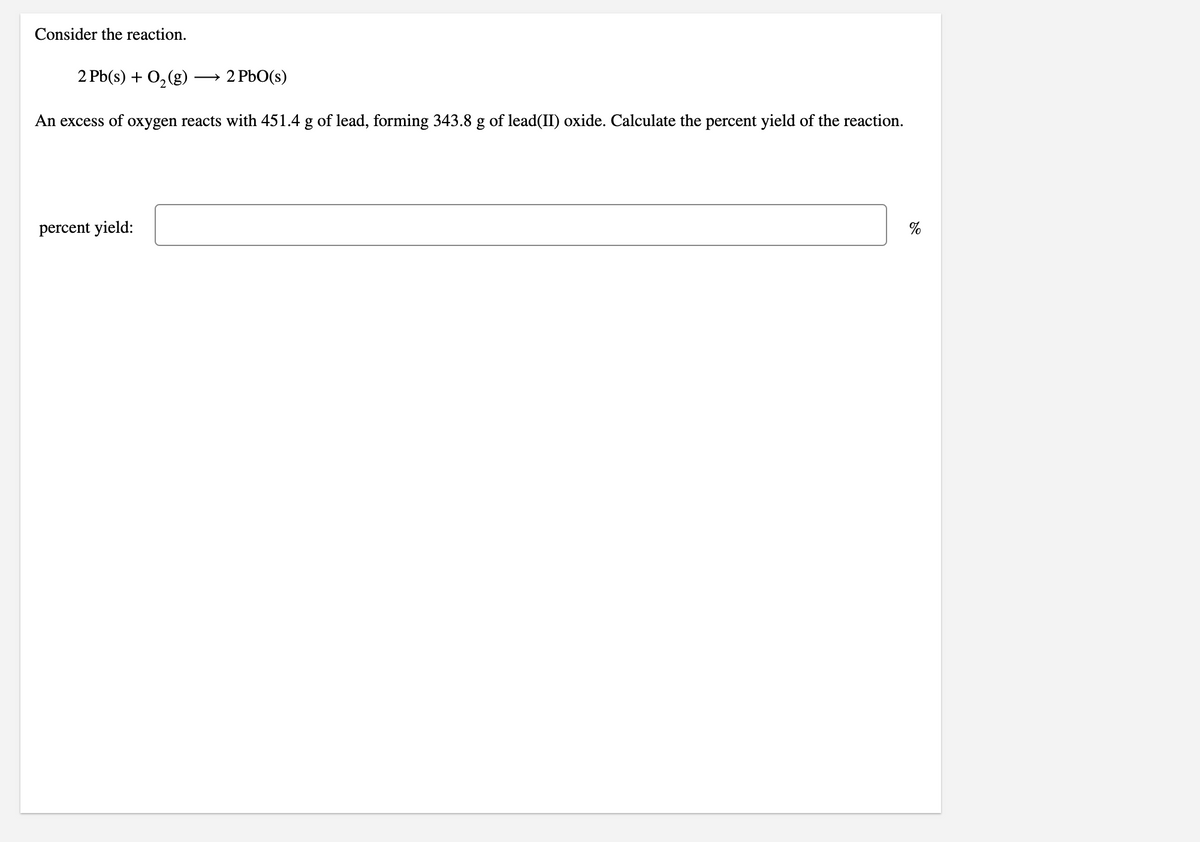
Transcribed Image Text:Consider the reaction.
2 Pb(s) + 0,(g)
→ 2 PbO(s)
An excess of oxygen reacts with 451.4 g of lead, forming 343.8 g of lead(II) oxide. Calculate the percent yield of the reaction.
percent yield:
%
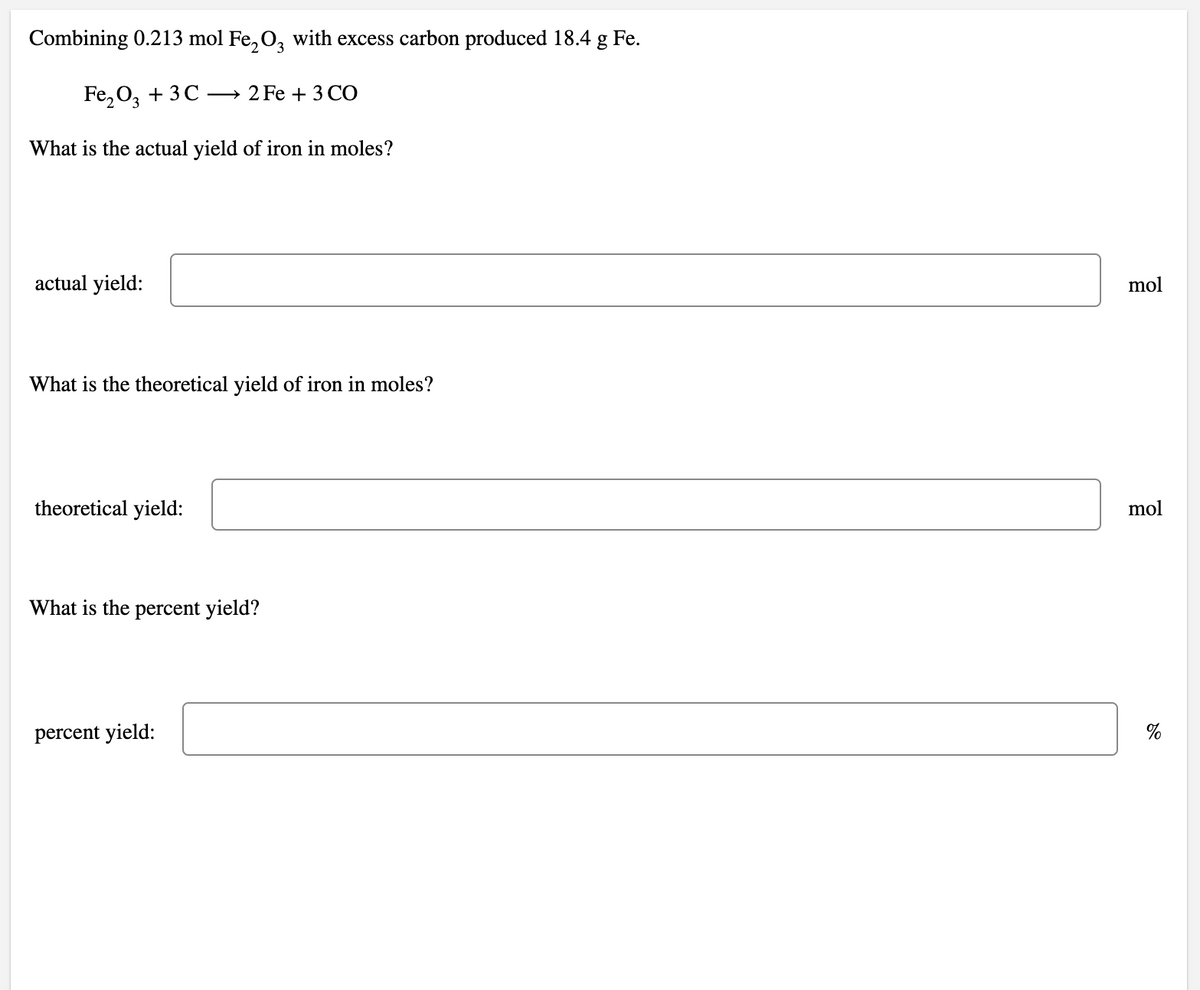
Transcribed Image Text:Combining 0.213 mol Fe, O, with excess carbon produced 18.4 g Fe.
Fe, O, + 3C — 2 Fe + 3CО
What is the actual yield of iron in moles?
actual yield:
mol
What is the theoretical yield of iron in moles?
theoretical yield:
mol
What is the percent yield?
percent yield:
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning