Which of the following is completely true regarding the titration of iron oxalate salt with EDTA? It was a redox titration, and the color changed from purple to yellow due to the disappearance of the purple reactant. It was a chelometric titration, and the color changed from purple to yellow, due to the disappearance of the purple reactant. It was a redox titration, and the color changed from yellow to pink due to the indicator added. It was a redox titration, and the color changed from yellow to pink due to the color of EDTA. It was a chelometric titration, and the color changed from clear to pink, due to the indicator added.
Which of the following is completely true regarding the titration of iron oxalate salt with EDTA? It was a redox titration, and the color changed from purple to yellow due to the disappearance of the purple reactant. It was a chelometric titration, and the color changed from purple to yellow, due to the disappearance of the purple reactant. It was a redox titration, and the color changed from yellow to pink due to the indicator added. It was a redox titration, and the color changed from yellow to pink due to the color of EDTA. It was a chelometric titration, and the color changed from clear to pink, due to the indicator added.
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.3QAP
Related questions
Question
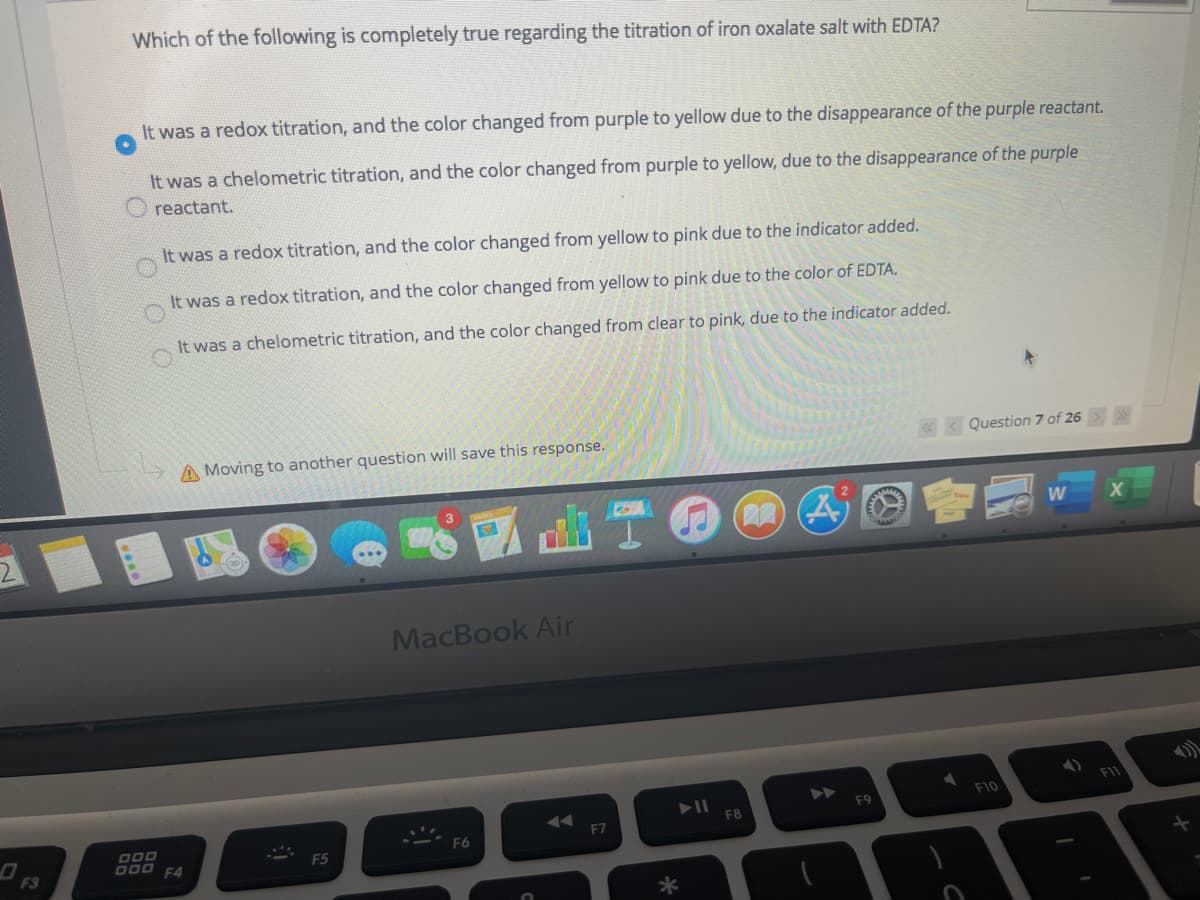
Transcribed Image Text:Which of the following is completely true regarding the titration of iron oxalate salt with EDTA?
It was a redox titration, and the color changed from purple to yellow due to the disappearance of the purple reactant.
It was a chelometric titration, and the color changed from purple to yellow, due to the disappearance of the purple
reactant.
It was a redox titration, and the color changed from yellow to pink due to the indicator added.
It was a redox titration, and the color changed from yellow to pink due to the color of EDTA.
It was a chelometric titration, and the color changed from clear to pink, due to the indicator added.
A Moving to another question will save this response.
«< Question 7 of 26 >>
MacBook Air
F10
F9
F8
F7
000
D00 F4
F6
F5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

