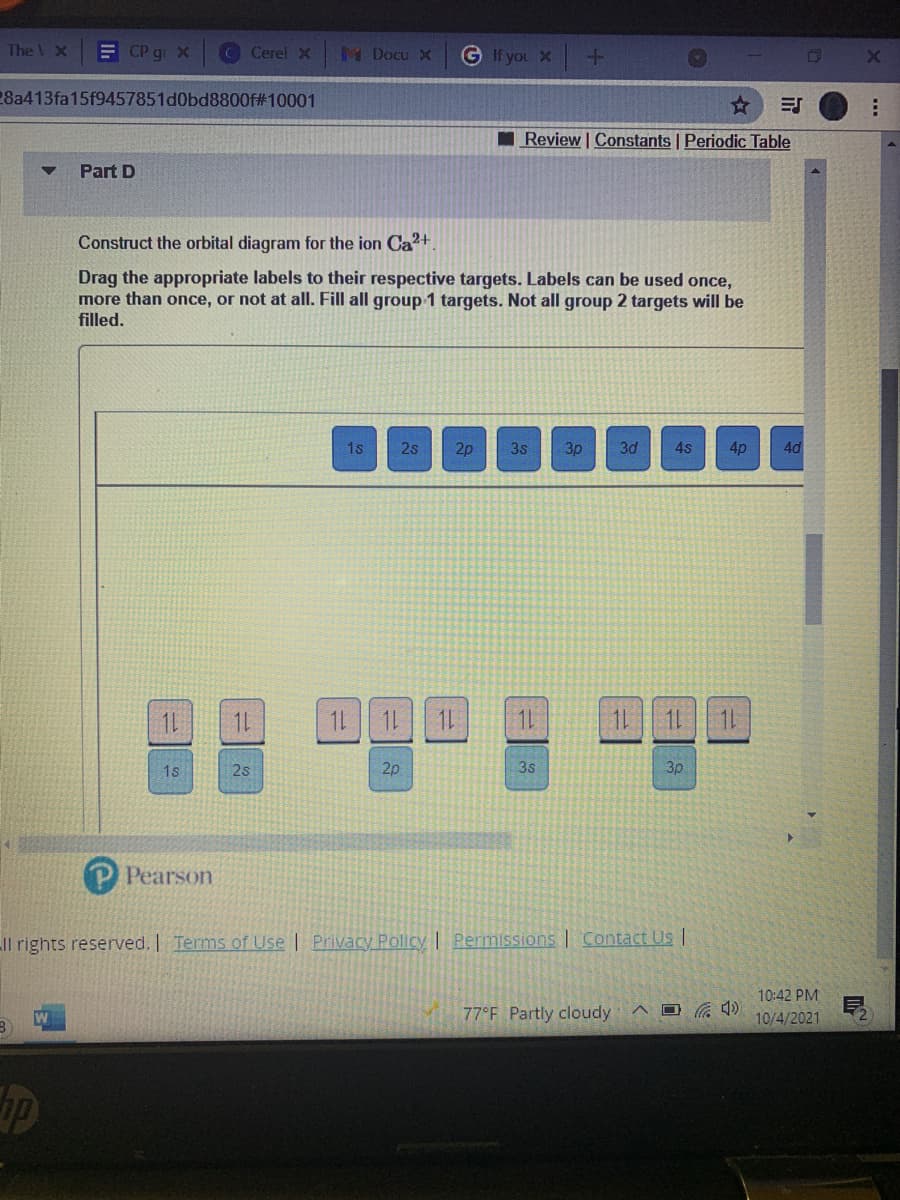
Construct the orbital diagram for the ion Ca2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Fill all group 1 targets. Not all group 2 targets will be filled. 1s 2s 2p 3s 3p 3d 4s 4p 4d 1L 1L 1L 1L 1L 1L 1L 11 1L 1s 2s 2p 3s 3p
Construct the orbital diagram for the ion Ca2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Fill all group 1 targets. Not all group 2 targets will be filled. 1s 2s 2p 3s 3p 3d 4s 4p 4d 1L 1L 1L 1L 1L 1L 1L 11 1L 1s 2s 2p 3s 3p
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 33PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Question
Transcribed Image Text:The V X
E CP gi x
C Cerel
M Docu X
G If you X
28a413fa15f9457851d0bd8800f#10001
I Review | Constants Periodic Table
Part D
Construct the orbital diagram for the ion Ca2+.
Drag the appropriate labels to their respective targets. Labels can be used once,
more than once, or not at all. Fill all group 1 targets. Not all group 2 targets will be
filled.
1s
2s
2p
3s
3p
3d
4s
4p
4d
1L
1L
1L 1L 1L
1L
1L 11 1
1s
2s
2p
3s
3p
P Pearson
Il rights reserved. Terms of Use | Privacy Policy | Permissions | Contact Us |
10:42 PM
77°F Partly cloudy
10/4/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning