Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.13QAP
Related questions
Question
This is grade 11 chemistry so don't give me an answer above that level please.
consider the ionization energy data in the graph. Which group does this atom belong to?
*please show your explanations so I understand better, and if you reply with a good answer and fast I will give an excellent rating. thank you
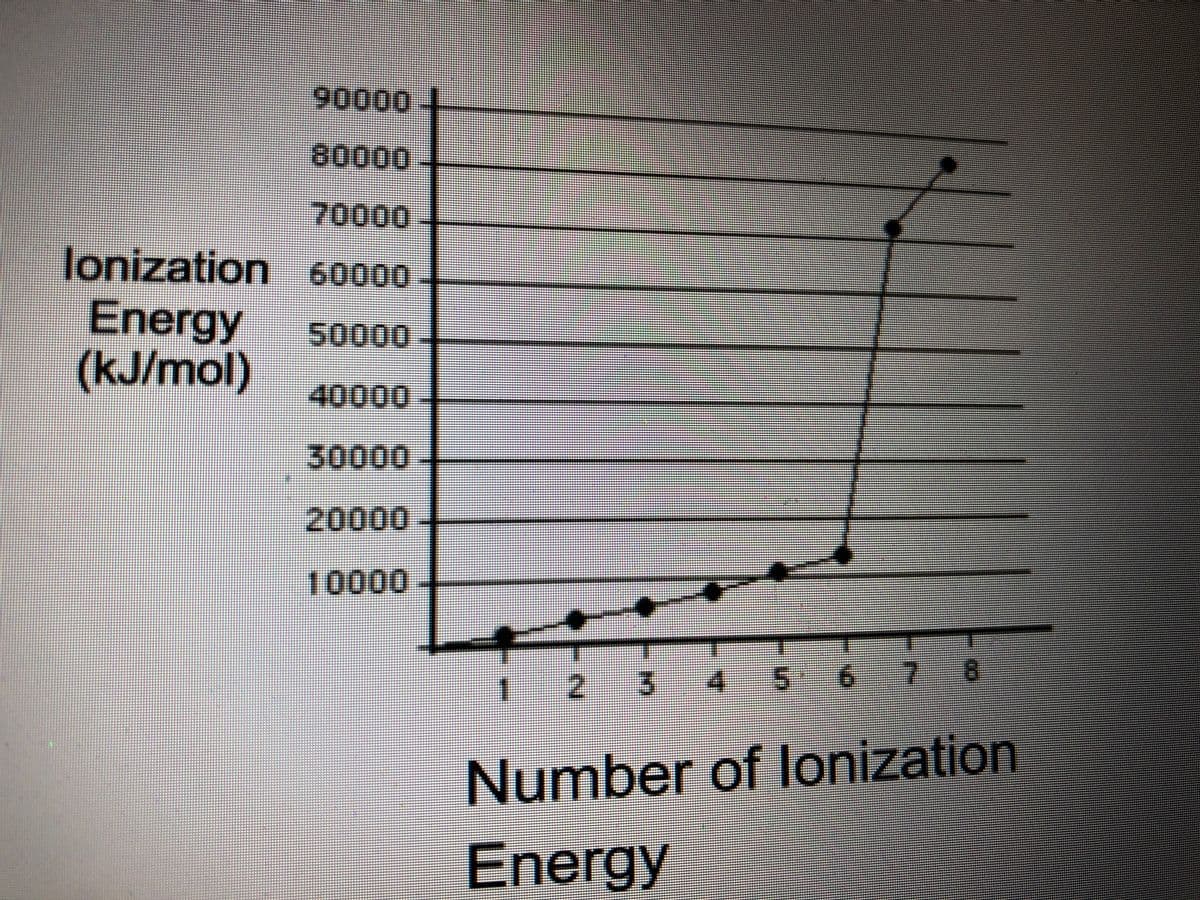
Transcribed Image Text:90000
80000
70000
lonization 60000
Energy
(kJ/mol)
50000
40000
30000
20000
10000
|2 3
4.
5 6
8
Number of lonization
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
