Construction of a voltaic cell Consider a iron-silver voltaic cell that is constructed such that one half-cell consists of the iron, Fe, electrode immersed in a Fe(NO3)3 solution, and the other half-cell consists of the silver, Ag electrode immersed in a AgNOz solution. The two electrodes are connected by a copper wire. The Fe electrode acts as the anode, and the Ag electrode acts as the cathode. To maintain electric neutrality, you add a KNO3 salt bridge separating the two half-cells. Use this information to solve Parts B, C, and D. Part B The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the iron-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s). (), or (g) for solid, liquid, or gas, respectively. Use (aq) for an aqueous solution. Do not include phases for electrons. Express your answer as a chemical equation. > View Available Hint(s) ΑΣφ 3Fe(s)→Fe* (aq)+2e' OA chemical reaction does not occur for this question. Submit Previous Answers X Incorrect; Try Again; 2 attempts remaining Part C The half-cell is a chamber in the voltaic cell where one half-cell is the site of an oxidation reaction and the other half-cell is the site of a reduction reaction. Type the half-cell reaction that takes place at the cathode for the iron-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s). (1). or (g) for solid, liquid, or gas, respectively. Use (ag) for an aqueous solution. Do not include phases for electrons. Express your answer as a chemical equation.
Construction of a voltaic cell Consider a iron-silver voltaic cell that is constructed such that one half-cell consists of the iron, Fe, electrode immersed in a Fe(NO3)3 solution, and the other half-cell consists of the silver, Ag electrode immersed in a AgNOz solution. The two electrodes are connected by a copper wire. The Fe electrode acts as the anode, and the Ag electrode acts as the cathode. To maintain electric neutrality, you add a KNO3 salt bridge separating the two half-cells. Use this information to solve Parts B, C, and D. Part B The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the iron-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s). (), or (g) for solid, liquid, or gas, respectively. Use (aq) for an aqueous solution. Do not include phases for electrons. Express your answer as a chemical equation. > View Available Hint(s) ΑΣφ 3Fe(s)→Fe* (aq)+2e' OA chemical reaction does not occur for this question. Submit Previous Answers X Incorrect; Try Again; 2 attempts remaining Part C The half-cell is a chamber in the voltaic cell where one half-cell is the site of an oxidation reaction and the other half-cell is the site of a reduction reaction. Type the half-cell reaction that takes place at the cathode for the iron-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s). (1). or (g) for solid, liquid, or gas, respectively. Use (ag) for an aqueous solution. Do not include phases for electrons. Express your answer as a chemical equation.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.32QP: You have 1.0 M solutions of Al(NO3)3 and AgNO3 along with Al and Ag electrodes to construct a...
Related questions
Question
100%
Cannot Figure out either of the questions
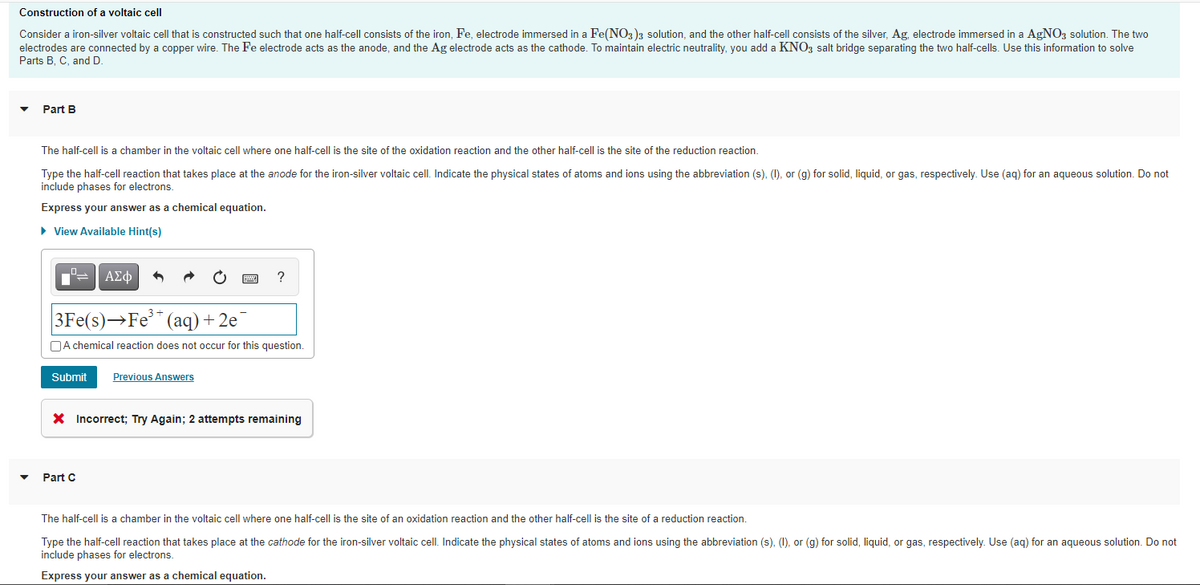
Transcribed Image Text:Construction of a voltaic cell
Consider a iron-silver voltaic cell that is constructed such that one half-cell consists of the iron, Fe, electrode immersed in a Fe(NO3)3 solution, and the other half-cell consists of the silver, Ag, electrode immersed in a AgNOz solution. The two
electrodes are connected by a copper wire. The Fe electrode acts as the anode, and the Ag electrode acts as the cathode. To maintain electric neutrality, you add a KNO3 salt bridge separating the two half-cells. Use this information to solve
Parts B, C, and D
Part B
The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction.
Type the half-cell reaction that takes place at the anode for the iron-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s), (), or (g) for solid, liquid, or gas, respectively. Use (ag) for an aqueous solution. Do not
include phases for electrons.
Express your answer as a chemical equation.
• View Available Hint(s)
ΑΣφ
?
3Fe(s)→Fe** (aq)+2e
OA chemical reaction does not occur for this question.
Submit
Previous Answers
X Incorrect; Try Again; 2 attempts remaining
Part C
The half-cell is a chamber in the voltaic cell where one half-cell is the site of an oxidation reaction and the other half-cell is the site of a reduction reaction.
Type the half-cell reaction that takes place at the cathode for the iron-silver voltaic cell. Indicate the physical states of atoms and ions using the abbreviation (s), (1), or (g) for solid, liquid, or gas, respectively. Use (aq) for an aqueous solution. Do not
include phases for electrons.
Express your answer as a chemical equation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning