D Question 20 In the diagram below, enzymes 1. 2, and 3 catalyze the reactions with substrates A, B, and C, respectively. Molecule D is an allosteric inhibitor to enzyme 2. Which of the following is another mechanism necessarily caused by substrate D? A B D O Cooperative rate regulation Irreversible inhibition O Noncompetitive inhibition O Negative feedback regulation O Coupled enzyme enhancement
D Question 20 In the diagram below, enzymes 1. 2, and 3 catalyze the reactions with substrates A, B, and C, respectively. Molecule D is an allosteric inhibitor to enzyme 2. Which of the following is another mechanism necessarily caused by substrate D? A B D O Cooperative rate regulation Irreversible inhibition O Noncompetitive inhibition O Negative feedback regulation O Coupled enzyme enhancement
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
Help me please

Transcribed Image Text:Question 20
In the diagram below, enzymes 1. 2. and 3
catalyze the reactions with substrates A, B,
and C, respectively. Molecule D is an allosteric
inhibitor to enzyme 2. Which of the following
is another mechanism necessarily caused by
substrate D?
A
C
O Cooperative rate regulation
O Irreversible inhibition
O Noncompetitive inhibition
O Negative feedback regulation
O Coupled enzyme enhancement
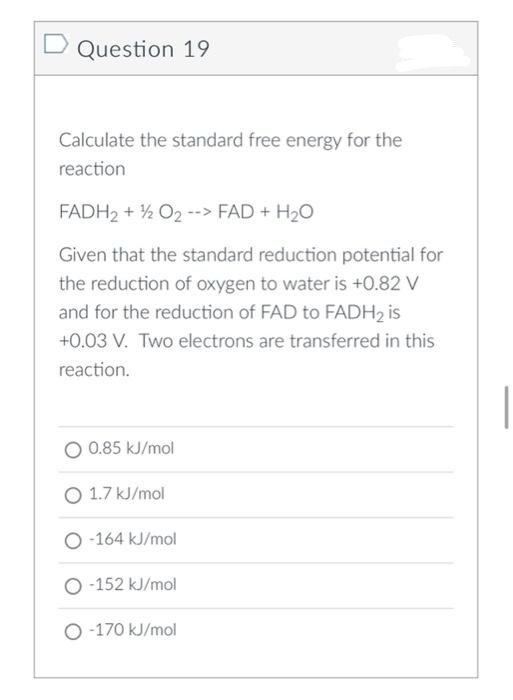
Transcribed Image Text:Question 19
Calculate the standard free energy for the
reaction
FADH2 + ½ O2 --> FAD + H2O
Given that the standard reduction potential for
the reduction of oxygen to water is +0.82 V
and for the reduction of FAD to FADH2 is
+0.03 V. Two electrons are transferred in this
reaction.
O 0.85 kJ/mol
O 1.7 kJ/mol
O -164 kJ/mol
O -152 kJ/mol
O -170 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON