Data: Trial 1 Trial 2 Trial 3 Leave Blank. Leave Blank. 10.73g Mass of Beaker 25.니c Leave Blank. Leave Blank. Temp of Water Mass of Beaker + Water 3490g 34.45g 34.89 Mass of Water Volume of Water 2.0x101 mL 2.0x101 mL 2.0x101 mL
Q: Which method should lead to greater precision? Which method actually is more precise? Explain
A: Method 1 relative average deviation is calculated as,
Q: ) bo rumeter heads 104 k Pa. express atmusphenc Phessure lof each unitt? la)em Hg= b.) Psi %3D
A:
Q: 2.40 mole H2O into liters (d=.9998 g/cm³)
A: We have to predict the volume of water in liters using dimensional analysis.
Q: Three liquid substances of the same mass and have an S.G. of 1.50, 0.835 and 1.2 were being mixed to…
A: Specific gravity of liquid 1 = 1.50 Specific gravity of liquid 2 = 0.835 Specific gravity of liquid…
Q: Lab Data Mean Mass (g) Mean Volume (mL) Mean Density (g/mL) Slope (g/mL) 6.951 10.710 1.3 8.2 18.512…
A:
Q: I need help in calculating the total mass of the unknown #2 sample of copper. The following chemical…
A: The given equations are: 2 Cu2+ + 4 I- ----> 2 Cus + 2 I2 I2 + 2 S2O3-2 ----> 2 I- +S4O6-2…
Q: A student performed an experiment to calculate the density of 10% aqueous solution of sodium…
A: • density = mass /volume
Q: Determine the specific gravity of the sample liquid given the following information: Weight of…
A:
Q: A 10.0-mL sample of alcohol is pipetted into a flask with stopper. The mass is found by difference…
A: The density of the alcohol is calculated as shown below where d, m, and V are the density, mass, and…
Q: Question 8 A student was experimenting with density to find the percent composition of an unknown…
A: Given that, the temperature of the experiment is T = 20°C = (273+20) K = 293 K. The mass of the…
Q: Perform each of the following conversions be sure to set up proper conversion factor in each case A)…
A: Conversion of 1.75 miles to kilometers should be given.Unit conversion from mi to in km can be done…
Q: Least count of the balance 0.1 g 1 21.0 24.0 Mass of solid, reading 1 25 g 2 19.0 21.0 Mass…
A: Concept: Density = Mass/ Volume To complete the table using the given experimental data
Q: Gravimetric means (volume measurement jor: the component to be analyzed .A True .B False .C .D
A:
Q: True Value 124 g True Value 1.75 lb 1. 6. Trial # 1 120 g Trial # 1 2.75 lb Trial # 2 121 g Trial #…
A: 1. Mean of the experimental value = (120+120+121)/3 = 120.3 True value = 124g Percentage error = […
Q: 1. (a) (7.59 g - 590 mg) + (6.55 mL+ 4.51 × 10-' cm') g/cm³ (b) 0.500 kg/m³ µg/µL (use dimensional…
A: 1 mg = 1000 g 1 mL = 1 cm3 1 kg = 109 μg 1 m3 = 109 μL
Q: Acetonitrile (ACN) is a common solvent utilized in to preparation of different classes of organic…
A: Step 1) ACN or acetonitrile is a simple organic nitrile. it is commonly abbreviated as methyl…
Q: Here is a plot of Ruth's data based on the table of data for the class: Table 1: Class Average…
A: From the reaction stoichiometry it is evident that 1 bicarbonate releases 1 carbon dioxide molecule,…
Q: Separation of a 3:1 Mixture Table view List view Recovered Sand Recovered Water Mass of Empty…
A: As per given experiment:- 1)Mass of seperated sand = 1.145 g. 2) Mass of seperated water = 2.455 g.…
Q: A student dissolves 12.4g of ammonium nitrate (NH4NO3) in 200.g of water in a well-insulated open…
A: Given, Mass of Ammonium nitrate = 12.4 g Mass of water = 200.0 g Temperature of water falls from 21◦…
Q: Calculate % difference between trials 1 and 2. Your value MUST be below 2% difference. Then…
A: The data given is,
Q: 2.4x10-3 mol Sb Express your answer using two significant figures. m = Submit Request Answer bo
A: Detail mathematical calculation is shown below
Q: a) The distance between Los Angeles and Washington D.C. is 4,297 kilometers. Express this distance…
A: The SI system which is basically the international system for the unit prefixes to be used all over…
Q: Pls help me with the density of water, average density of water, standard deviation, and percent…
A:
Q: A 55.9kg person displaces 67.2l of water when submerged in water tank. What is the density of the…
A: Given: Mass of person = 55.9 Kg Volume of water displaces = 57.2 L
Q: A 50-mL volumetric flask measures a volume of 50.00 mL. In this experiment, we want to show that…
A: Hello, Since the question contains multiple subparts, the first three subparts are solved. In case…
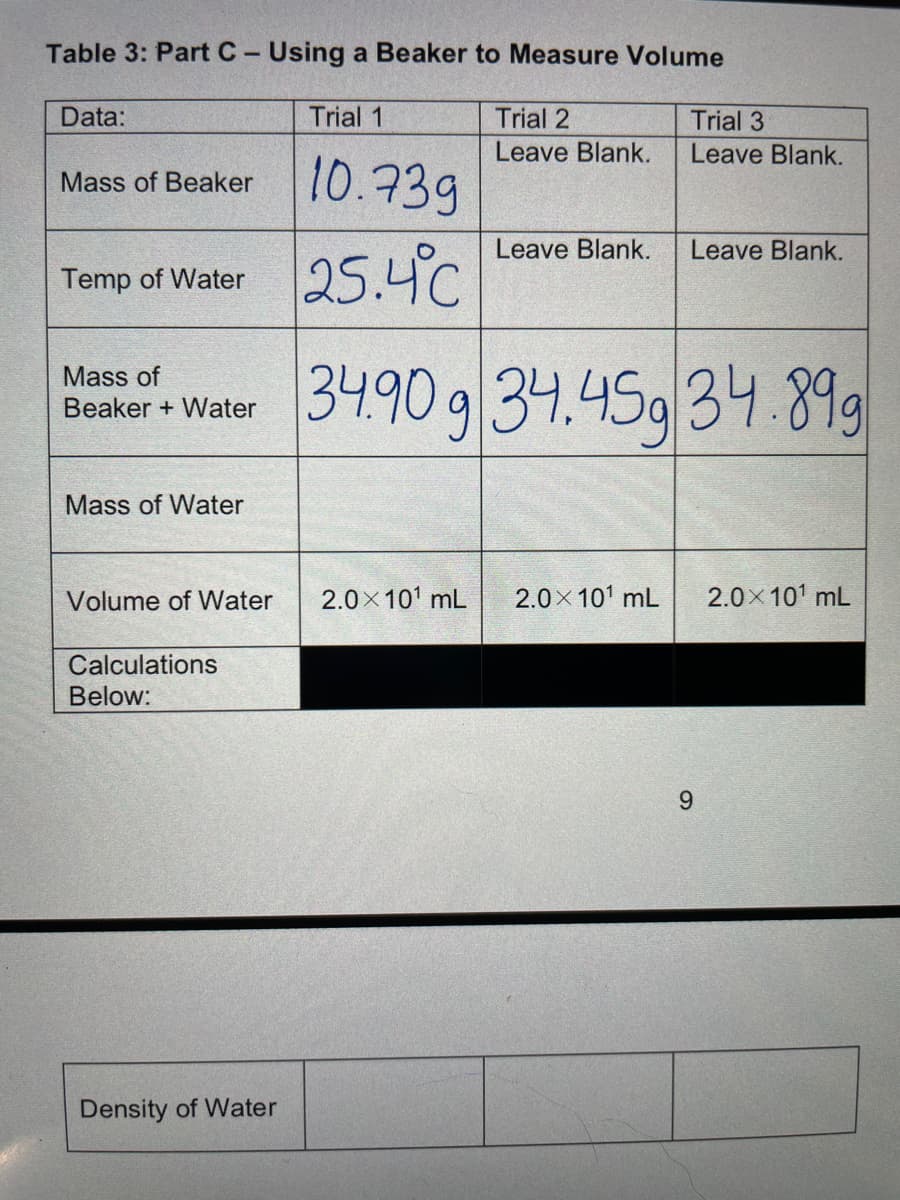
Q: Table 3: Part C-Using a Beaker to Measure Volume Data: Trial 1 Trial 2 Leave Blank. Trial 3 Leave…
A: Density is mass per unit volume and it is calculated by the formula Density = Mass used / Volume…
Q: metric system conversions can you help explain how to get to answer for the following problems 0.2…
A: Here 0.2 deciliter (dL) is converted into liter (L)As 1 deciliter (dL) = 0.1 liter (L)So 0.2…
Q: A 0.3256 gram sample sodiun oxalate ( GEW= 67) was used for the standardization of KMn04 and…
A: Given, Mass of sodium oxalate in g = 0.3256 g Gram equivalent weight of sodium oxalate = 67…
Q: Two students each measured the density of a liquid three times and recorded the following data in…
A: Here, we have to find out which solution is more accurate and precise. Given: true density = 2.085…
Q: ge.com/course.html?courseld%3D15807691&OpenVellumHMAC=D4813aeeccd169993069b9dc37c5b2cc <Chapter 1…
A: The conversion factors of grams to microgram, megagram, milligram and nanogram are given as follows:
Q: In a similar experiment, a pair of students was asked to measure out 10.00 mL of water using a 25-mL…
A: Accuracy refers to closeness to the actual value. Precision refers to closeness among observed…
Q: YouTube Maps MyCSU Columbus... - B Homepage - Georg... W Microsoft Of MATTER mass percent…
A: Solution is made up of solute and solvent. Solute is present in small amount and solvent is present…
Q: Mass of solvent in kilograms. Hint: Mass solvent in grams was 151.5 Use the metric definition 1…
A: Given: Mass of solvent in g = 151.5 g. Since 1 Kg = 1000 g.
Q: Table 1: Part A- Using a 3.0 mL Plastic Pipette to Measure Volume Data: Trial 1 Trial 2 Trial 3…
A: Given: Mass of beaker for trail 1 = 24.78 g Mass of beaker + water for trail 1 = 26.89 g Mass of…
Q: Lab of Density and specific gravity
A:
Q: -1 A certain liquid X has a normal freezing point of -9.00 °C and a freezing point depression…
A: Freezing point of a liquid is defined as the temperature at which it gets converted to solid.…
Q: Table 2: Part B- Using a Graduated Cylinder to Measure Volume Data: Trial 1 Trial 2 Trial 3 Leave…
A: Percent Error:- It is basically the percent deviation from the actual value of the experimental…
Q: Quantity Standard Millimeter Mercury Cadnaen mm Hg) Torr atmosphere(atm) 1 Pascal( Pa) nienimateh sl…
A: Unit is defined as the quantity which has a fixed magnitude. Many units of a physical quantity are…
Q: Determine the mass of each sample or portion of water added to the 100 mL beaker for each measuring…
A: We have to calculate the mass of each portion of water added to the 100 mL of beaker for each…
Q: 2- Measuring the Mass of Liquids. Determination of the Density of a Liquid. Trial 2 Trial 1 Mass of…
A: Specific gravity is relative density, that is, density of sample divided by density of water…
Q: 1. Fifita uses a thermometer and finds the boiling põint öf consecutive experimental measurements.…
A: Since you have posted more than one question, we are solving first for you. To solve the remaining…
Q: Given: wt NaOH = 10.0g Density of NaOH= 2.16 g/ml Wt. H2O = 200g Density of H2O=…
A: Molarity of a solution may be calculated by dividing the number of moles of solute by the volume of…
Q: Density of Unknown Liquid TRIAL 1 TRIAL 2 Mass of beaker (g) 29.555 g N/A Mass of beaker and liquid…
A: Analytical techniques give importance to accuracy and precision. Errors can be personal or…
Q: S F H. Name: Date lab performed: Partner(s) name: Date due: Measurements: IDensity of a Saline…
A: Mass of empty beaker = M(empty) = 31.343 g Mass of beaker after 1st addition of saline = M (1st) =…
Q: Data and Observations: Data Table Mass Volume Solution A-1 6.30009 4,25009 5.00ML 5.00 ML Solution…
A:
Q: mol A chemist must prepare 475. mL of 1.00 M aqueous aluminum chloride (AICI,) working solution.…
A:
Q: Calculating Percent Error Use Student A’s density data in Table 2.3 to calculate the percent error…
A: Since the data for student A is asked, thus percent error for student A has been calculated only.…
Q: A man wanted to prepare a fertilizer solution for his garden. The instructions were dissolve 7 ml of…
A: The required concentration of fertilizer is = 7 mL in four-gallon The volume of the container is =…
Q: Gold occurs in the ocean in a range of concentrations of 0.1 to 2 mg gold per ton of seawater. Near…
A: Given recovery = 64℅ 1 troy = 31.1g
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- The atmosphere is a highly complex gaseous mixture that sustains life on Earth. Approximately 99% of the air is composed of nitrogen (N2) and oxygen (O2). The remaining 1% is made up of a variety of other gases, including carbon monoxide (CO), hydrogen (H2), and ammonia (NH3), among many others. Because most of the gases that comprise the atmosphere are present at very low levels (<0.002%), their quantities are often expressed in parts per million (ppm) or parts per billion (ppb) rather than as a percent. Ozone (O3) is found in the troposphere at 2.5×10−6%. Convert this value to parts per million. [O3]= _______ ppm The atmosphere contains 2.9×10−7%2.9×10−7% nitrogen dioxide (NO2). Convert this value to parts per billion. [NO2]= _______ ppb Atmospheric methane (CH4) is present at 1983 ppb. Convert this value to a percentage. [CH4]= _______ %A 0.2121-g sample of an organic compound was burned in a stream of oxygen, and the CO2 produced was collected in a solution of barium hydroxide Ba(OH)2. if 0.6006 g of BaCO3( 197.3 g/mol) was formed as precipice, Calculate: (write the answer without unit with 2 digits after decimal point) 1) mMoles CO2 2) the percentage of carbon(12 g/mol) in the sample (%C) CO2 + Ba(OH)2 BaCO3 + H2OSolve for the the Dimensional Analysis (Predicted Volume of NaOH) Using the Data below:Given : Balanced Chemical Equation:NaOH (aq) + KHC8H4O4 (aq) → KNaC8H4O4 (aq) + H2O (l) Note: KHC8H4O4 = KHP Standardization of Sodium Hydroxide- Primary Standard used: 204.22 g/mol- Formula Mass of Primary Standard: Potassium hydrogen phthalate (KHC8H4O4)- % Purity of Primary Standard: 99.5% TRIALS 1 2 3 Actual weight of Std. KHP (g) 0.8026 0.8026 0.8021 Corrected weight of KHP (g) based on % purity of primary std. 0.7986g 0.7992g 0.7981g
- A company has just patented a new synthetic alcohol for alcohol beverages. You analyse asample of the new product by placing a small volume of it in a round-bottomed flask with avolume of 612.0 mL and an evacuated mass of 131.918 g. You submerge the flask in a waterbath at 100°C and allow the volatile liquid to vaporize. You then cap the flask and remove itfrom the water bath. You weight it and determine the mass of the vapour in the flask to be0.9198 g. what is the molar mass of the volatile liquid, and what does it mean with regard tothe new product? (Assume the pressure is 1 atm)Ammonia is synthesized from nitrogen and hydrogen in the following reaction: N2(g) + 3 H2(g) ⇔ 2 NH3(g)At 500 oC, Given that [NH3] = 0.319 M, [N2] = 0.216 M, and [H2] = 0.459 M, find Keq. (Answer to 4 decimal places)Air has an average molar mass of 29.0 g/mol. The density of air at 0.99atm and 30.0oC is:(Ignore significant figures for this problem.)Select one:a. 29.0 g/Lb. 39.8g/mLc. 1.15g/Ld. 1.37g/mLe. 11.7g/L
- kindly help me with this problem solve as neatly as possible and show completesolution. Round your final answer to 4 decimal places and box / highlight all final answers. all values must include proper units with properconversion if needed in your solution. Please follow the format Given,Required,Solution thank you! 1. The following data were taken in measuring the molecular weight of a certain gas by theRegnault’s Method: o Wt of evacuated bulb : 32.5050 go Wt of bulb+gas : 33.5417 go Wt of bulb+water : 385.31 go Temperature : 25 OCo P : 760 mm HgFind the Molecular Weight of the gas. What could have been this gas?The volume of an automobile tire is 2.5 * 10 ^ - 2 * m ^ 3 pressure of the air in the tire is 2.5 atm and the temperature is 280 what is is the mass of air grams ? The mean molecular mass of air is 29g 1 atm = 1.01 * 10 ^ 5 * p_{a} ; Calculate to 2 decimals.kindly help me with this problem solve as neatly as possible and show completesolution. Round your final answer to 4 decimal places and box / highlight all final answers. all values must include proper units with properconversion if needed in your solution. Please follow the format Given,Required,Solution thank you! Additional-the given temperature is 0 degrees 2. The densities of Methane at 0C were measured at several pressures with the followingresults: Pressure (atm) Density(g/L) 1⁄4 0.17793 1⁄2 0.35700 3⁄4 0.53740 1 0.71707 Find the Molecular Weight of Methane
- 5.86 A student mixes 88.6 g of water at 74.3°C with 57.9 gof water at 24.8°C in an insulated flask. What is the finaltemperature of the combined water?A consumer upset with the latest trend of postal rate increases has decided to try to send letters by balloon even though they may not reach their intended destinations. A 6.00×104 cm36.00×104 cm3 gas-filled balloon will provide enough lift for a 45.1 g45.1 g package to be accelerated upward at a rate of 2.80 m/s22.80 m/s2. For these circumstances, calculate the density of the gas the consumer fills the balloon with. The acceleration due to gravity is ?=9.81 m/s2g=9.81 m/s2 and the density of air is ?air=1.16 kg/m3ρair=1.16 kg/m3. Neglect the mass of the balloon material and the volume of the package.1. A 70 kg man expends 480 kcalkcal of energy per hour shoveling snow (note that kcal is just a unit of energy). The oxidation of organic nutrients such as glucose during metabolism liberates approximately 3.36 kcal of energy per gram of oxygen consumed. If air is 21% oxygen, what volume of air at STP is needed to produce enough energy for the man to clear snow from a walkway that requires 35 minutes of shoveling? (answer in L) 2. A steel cylinder of compressed argon with a volume of 0.4000L was filled to a pressure of 145atm at 10.°C. At 1.00 atm pressure and 25°C, how many 15.0 mL incandescent light bulbs could be filled from this cylinder?(Hint: find the number of moles of argon in each container.)

