Date Electron Structure 1. Draw the Bohr model for bydrogen witha single clectrn Show the l,2,) ergy level with the electron in the fiest or lowet level. Label the diagm 35 Li At Na Na 2. Use the diagram ahove to draw the deciron confipuratiom ef LLAr nd Na the nght de f the page. 3. Use the diagram above for LA Ar, nd Na nd wrie e eymbelic notation for the electon ture of Na. A, and Na Eample Mi l' 4. Use the diagram ahove o druw the electon configurtion of de k Na'nd CT alet de ig side of the page 5. U the dingrams abve for LA Ar, and Na and wrie te sybelie ion fer dhe eetet of Na, and C. 6What does he l qutunter sell ahout an clectron The Light Spectrem 1What color hes the lowet enmgy in the vible spectrum 2. What color has the bighest energy in the vile pecm | || | | | || |
Date Electron Structure 1. Draw the Bohr model for bydrogen witha single clectrn Show the l,2,) ergy level with the electron in the fiest or lowet level. Label the diagm 35 Li At Na Na 2. Use the diagram ahove to draw the deciron confipuratiom ef LLAr nd Na the nght de f the page. 3. Use the diagram above for LA Ar, nd Na nd wrie e eymbelic notation for the electon ture of Na. A, and Na Eample Mi l' 4. Use the diagram ahove o druw the electon configurtion of de k Na'nd CT alet de ig side of the page 5. U the dingrams abve for LA Ar, and Na and wrie te sybelie ion fer dhe eetet of Na, and C. 6What does he l qutunter sell ahout an clectron The Light Spectrem 1What color hes the lowet enmgy in the vible spectrum 2. What color has the bighest energy in the vile pecm | || | | | || |
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 40P: When metallic sodium is dissolved in liquid sodium chloride, electrons are released into the liquid....
Related questions
Question
100%
Please answer complete questions will give rating surely
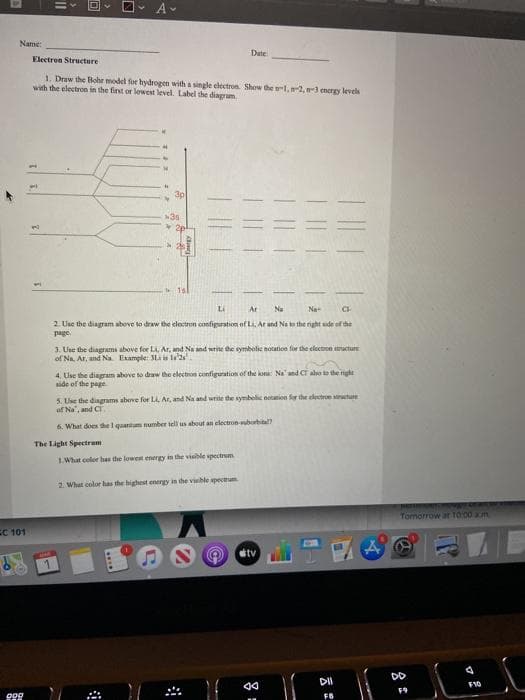
Transcribed Image Text:Name:
Date
Electron Structure
1. Draw the Bohr model for hydrogen with a single electron. Show the l,2, mergy leves
with the electron in the first or lowest level. Label the diagram
3p
1sl
Li
Ar
Na
Na
2. Use the diagram above to draw the clectron configpuration of La, At and Na to the right side of the
page
3. Use the diagrams above for LL Ar, and Na and write he symbolic notation for the electron structure
of Na. Ar, and Na Etample: Li is la
4. Use the diagram above so draw the electon configuration of the kona: Na' and CT also te he nighe
side of the page
5. Use the diagrams above for LA Ar, and Na and write the symbelic ntation for the electroe ture
of Na", and Cr.
6. What does the l quanitum number tell us about an electron-uborhital
The Light Spectrem
LWhat color has the lowent energy in the visble spectrum
2. What color has the highest energy in the visble spectrum.
NEYE
Tomorrow ar T0/00 am
EC 101
dtv
DD
DII
F9
FB
吕=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning