Decide whether each pair of elements in the table below will form an lonic compound. If they will, write the empirical formula of the compound formed in the space provided. empirical formula of lonic compound element 1 element 2 Forms lonic compound? lodine rubidium O ves O no ithium nuorine O ves no axygen rubidium yes no bromine OKygen yes O no
Decide whether each pair of elements in the table below will form an lonic compound. If they will, write the empirical formula of the compound formed in the space provided. empirical formula of lonic compound element 1 element 2 Forms lonic compound? lodine rubidium O ves O no ithium nuorine O ves no axygen rubidium yes no bromine OKygen yes O no
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 8STP
Related questions
Question
1
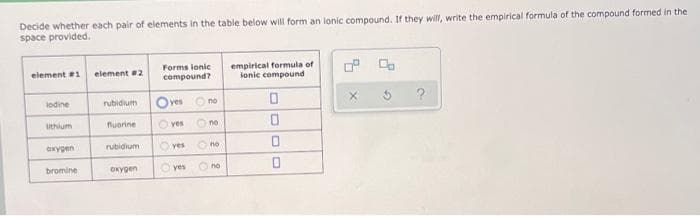
Transcribed Image Text:Decide whether each pair of elements in the table below will form an lonic compound. If they will, write the empirical formula of the compound formed in the
space provided.
Forms lonic
empirical formula of
ionic compound
element 1
element 2
compound?
lodine
rubidium
Oves O no
X.
ithium
fuorine
O ves
no
oxygen
rubidium
yes
no
bromine
OKygen
yes
O no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning



Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning